
The number of sigma and pi bonds in peroxydisulfuric acid is/are: [If answer is $13$ and $6$, represent as $136$]
Answer
586.8k+ views
Hint:Sigma bonds are formed by the head on overlapping whereas pi – bonds are formed with sideways overlapping. Single bond is a sigma bond, in double bond there is $1\sigma $ or $1\pi $ - bond and in triple bond there is $1\sigma $ and $2\pi $- bonds.
Complete step by step answer:
Peroxydisulfuric acid is the organic compound. It is also known as Marshall’s acid. It can also be said as the oxoacid of Sulphur.
Its chemical formula is ${H_2}{S_2}{O_8}$
Molar mass: $194.14g/mol$
Structure:
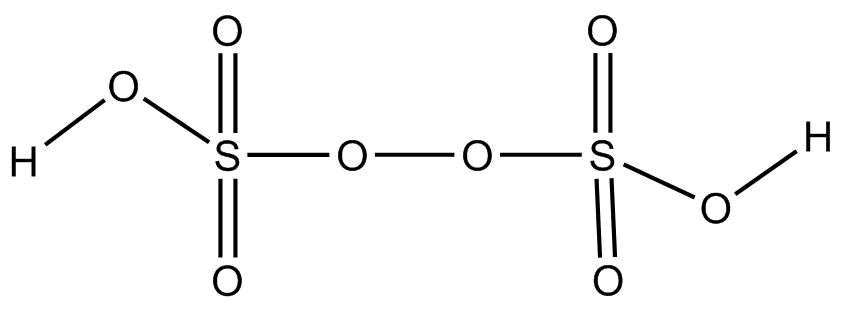
It is a colourless solid having no odor and is also soluble in water. The number of sigma or pi – bonds present in peroxodisulfuric acid are:
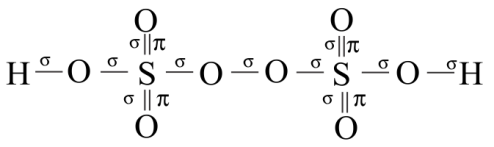
So, there are $11$ sigma bonds and $4$ pi – bonds in peroxodisulfuric acid.
As per the condition given in question the answer is $114$.
Additional information:
(A) Peroxodisulphuric acid and its salts are used as a source of hydrogen peroxide and due to this the large scale production of sulphuric acid is possible.
(B) It is also used as a hypo – eliminator in photography
(C) It can also be used as a strong oxidant.
Note:
The sigma and pi bonds count should be made wisely by keeping in mind that both the double and triple bonds have only $1\sigma $ bond and the rest of bonds in double and triple bonds are pi – bonds.
Complete step by step answer:
Peroxydisulfuric acid is the organic compound. It is also known as Marshall’s acid. It can also be said as the oxoacid of Sulphur.
Its chemical formula is ${H_2}{S_2}{O_8}$
Molar mass: $194.14g/mol$
Structure:

It is a colourless solid having no odor and is also soluble in water. The number of sigma or pi – bonds present in peroxodisulfuric acid are:

So, there are $11$ sigma bonds and $4$ pi – bonds in peroxodisulfuric acid.
As per the condition given in question the answer is $114$.
Additional information:
(A) Peroxodisulphuric acid and its salts are used as a source of hydrogen peroxide and due to this the large scale production of sulphuric acid is possible.
(B) It is also used as a hypo – eliminator in photography
(C) It can also be used as a strong oxidant.
Note:
The sigma and pi bonds count should be made wisely by keeping in mind that both the double and triple bonds have only $1\sigma $ bond and the rest of bonds in double and triple bonds are pi – bonds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




