
The number of resonating structures of aniline is:
(A) $2$
(B) $3$
(C) $4$
(D) $1$
Answer
567k+ views
Hint: Resonance effect arises due to the polarity that is produced in a molecule due to interaction between a lone pair and a $\pi $ bond electron. It can also arise when there is $\pi - \pi $ conjugated system i.e. interaction of two pi bonds between two adjacent atoms.
Complete step by step answer:
In chemistry, Resonance is the phenomenon in which a molecule can move its $\pi $ electrons in the system. These electrons delocalized themselves in the compound forming a conjugated system of $\pi $ electrons. This movement of the electrons cannot be exhibited with the help of one lewis structure, hence more than one structure is drawn to explain the delocalization. These structures are called resonating structures.
Resonance in chemistry is also used to explain the bonding in some particular molecules or ions by merging many structures, which are called canonical structures or resonance structures with the help of valence bond theory into a hybrid resonance which is called hybrid structures.
Before drawing the resonating structure of aniline, we have to first draw the structure of aniline; Aniline is an aromatic amine in which the $ - N{H_2}$ group is directly attached to $s{p^2}$ hybridized carbon of benzene ring. An aromatic amine such as aniline exhibits a resonance effect due to which the lone pair on nitrogen participates in delocalization with $\pi $ electrons of benzene ring system and is less available to be shared with other species.
The resonating structures of aniline can be drawn as:
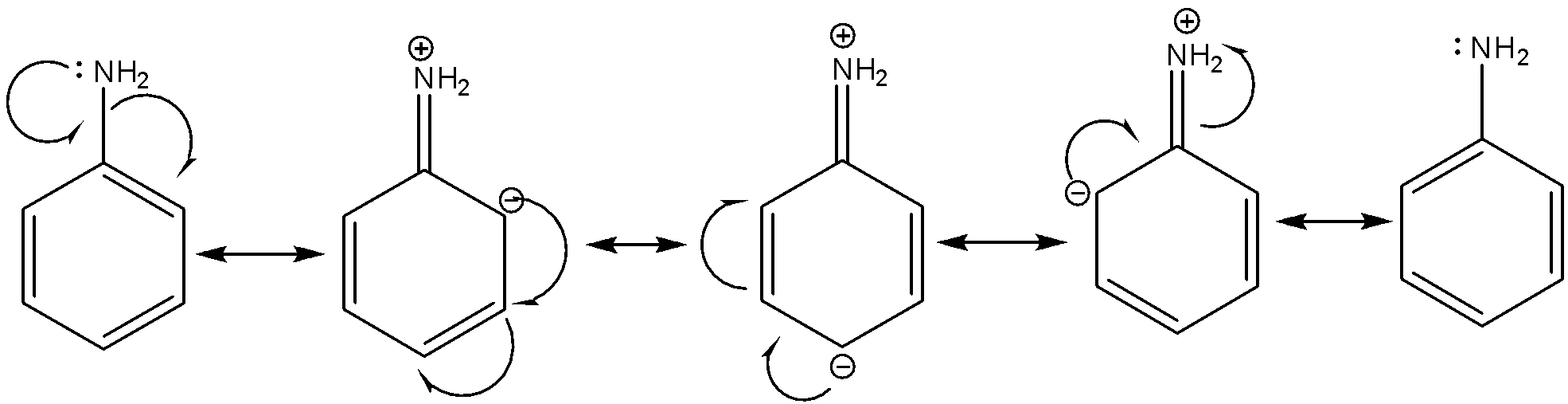
As, you can see that the total number of resonating structures of aniline is $5$.
Hence, option (D) is correct.
Note:
Aniline exists as a yellowish, brownish liquid which has a fish-like odour. It is a weak base. It is used in the rubber industry in the processing of rubber chemicals. Aniline is also used as a dyeing agent in cloth manufacturing, and as a pesticide and fungicides in agriculture.
Complete step by step answer:
In chemistry, Resonance is the phenomenon in which a molecule can move its $\pi $ electrons in the system. These electrons delocalized themselves in the compound forming a conjugated system of $\pi $ electrons. This movement of the electrons cannot be exhibited with the help of one lewis structure, hence more than one structure is drawn to explain the delocalization. These structures are called resonating structures.
Resonance in chemistry is also used to explain the bonding in some particular molecules or ions by merging many structures, which are called canonical structures or resonance structures with the help of valence bond theory into a hybrid resonance which is called hybrid structures.
Before drawing the resonating structure of aniline, we have to first draw the structure of aniline; Aniline is an aromatic amine in which the $ - N{H_2}$ group is directly attached to $s{p^2}$ hybridized carbon of benzene ring. An aromatic amine such as aniline exhibits a resonance effect due to which the lone pair on nitrogen participates in delocalization with $\pi $ electrons of benzene ring system and is less available to be shared with other species.
The resonating structures of aniline can be drawn as:

As, you can see that the total number of resonating structures of aniline is $5$.
Hence, option (D) is correct.
Note:
Aniline exists as a yellowish, brownish liquid which has a fish-like odour. It is a weak base. It is used in the rubber industry in the processing of rubber chemicals. Aniline is also used as a dyeing agent in cloth manufacturing, and as a pesticide and fungicides in agriculture.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




