
The number of pi bond(s) in a benzene molecule is/are:
A. 3 bonds
B. 6 bonds
C. 12 electrons
D. 1 bond
Answer
603.6k+ views
Hint: A pi bond is formed by the overlapping of orbitals laterally with the electron density concentrated above and below the plane of the nuclei. One pi bond is equivalent to two pi electrons.
Complete step by step answer:
Pi bonds are covalent chemical bonds which are formed by the sideways overlap of orbitals. The electron density is concentrated above and below the plane of the nuclei of the bonding atoms. The atomic orbitals formed have zero density at the shared nodal plane, passing through the two bonded nuclei. The same plane is also a nodal plane for the molecular orbital of the pi bond. Pi bonds can form in double and triple bonds but do not form in single bonds in most of the cases.
Pi bonds are usually weaker than sigma bonds. The bond energy of C-C double bond, which consists of one pi bond and one sigma bond is less than twice the bond energy of C-C single (sigma) bond.
Now, in the question, we need to find the number of pi bonds in benzene. The structure of benzene is:
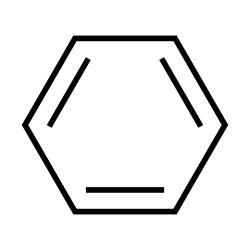
Clearly, benzene has three pi bonds or 6 pi electrons.
Hence, the correct answer is (A) 3 bonds.
Note: Remember that the above structure of benzene is one of its resonating structures. The real structure of benzene is a hybrid of the two resonating structures.
Complete step by step answer:
Pi bonds are covalent chemical bonds which are formed by the sideways overlap of orbitals. The electron density is concentrated above and below the plane of the nuclei of the bonding atoms. The atomic orbitals formed have zero density at the shared nodal plane, passing through the two bonded nuclei. The same plane is also a nodal plane for the molecular orbital of the pi bond. Pi bonds can form in double and triple bonds but do not form in single bonds in most of the cases.
Pi bonds are usually weaker than sigma bonds. The bond energy of C-C double bond, which consists of one pi bond and one sigma bond is less than twice the bond energy of C-C single (sigma) bond.
Now, in the question, we need to find the number of pi bonds in benzene. The structure of benzene is:

Clearly, benzene has three pi bonds or 6 pi electrons.
Hence, the correct answer is (A) 3 bonds.
Note: Remember that the above structure of benzene is one of its resonating structures. The real structure of benzene is a hybrid of the two resonating structures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




