
The number of peroxide bonds present in perxenate ion,${{\left[ Xe{{O}_{6}} \right]}^{4-}}$ is:
[A] 0
[B] 2
[C] 3
[D] 1
Answer
591.3k+ views
Hint: A peroxide bond has two oxygen atoms attached to itself through a single covalent bond. In perxenate ion, the geometry is octahedral and each oxygen atom is attached to the central ion, xenon.
Complete answer:
Peroxide is a chemical compound in which two oxygen atoms are linked to each other. The two oxygen atoms are attached to each other through a single, covalent bond. Example of the most common peroxide is ${{H}_{2}}{{O}_{2}}$.
Over here, we have the perxenate anion which is formed by the loss of four protons from perxenate which is ${{H}_{4}}Xe{{O}_{6}}$.
In the perxenate ion, as we can understand from the formula itself, the geometry is octahedral. There are eight valence shell electrons in xenon which means it can form eight bonding relationships with other atoms.
In perxenate anion, we have 6 oxygen atoms which means there are four oxygen atoms that will form a single bond with the centre ion and the other two will form double bonds in order to fully fill their valency.
Now, we can draw the structure of the perxenate ion as-
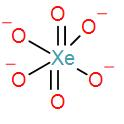
As we can see from the structure, all the oxygen atoms are forming covalent bonds with xenon. There is no oxygen-oxygen single bond, which means there is no peroxide bonding in the perxenate ion.
Therefore, the correct answer is option [A] 0, zero.
Note:
A peroxide bond is not very strong. As we have discussed above, in peroxide bonds two oxygen atoms are linked to each other through a single bond and we know that oxygen is an electronegative element. If two oxygen atoms are bonded to each other, there will be high electron-electron repulsion, hence makes the peroxide bonding weak.
Complete answer:
Peroxide is a chemical compound in which two oxygen atoms are linked to each other. The two oxygen atoms are attached to each other through a single, covalent bond. Example of the most common peroxide is ${{H}_{2}}{{O}_{2}}$.
Over here, we have the perxenate anion which is formed by the loss of four protons from perxenate which is ${{H}_{4}}Xe{{O}_{6}}$.
In the perxenate ion, as we can understand from the formula itself, the geometry is octahedral. There are eight valence shell electrons in xenon which means it can form eight bonding relationships with other atoms.
In perxenate anion, we have 6 oxygen atoms which means there are four oxygen atoms that will form a single bond with the centre ion and the other two will form double bonds in order to fully fill their valency.
Now, we can draw the structure of the perxenate ion as-

As we can see from the structure, all the oxygen atoms are forming covalent bonds with xenon. There is no oxygen-oxygen single bond, which means there is no peroxide bonding in the perxenate ion.
Therefore, the correct answer is option [A] 0, zero.
Note:
A peroxide bond is not very strong. As we have discussed above, in peroxide bonds two oxygen atoms are linked to each other through a single bond and we know that oxygen is an electronegative element. If two oxygen atoms are bonded to each other, there will be high electron-electron repulsion, hence makes the peroxide bonding weak.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




