
The number of $P - OH$ bonds and the oxidation state of phosphorus atom in pyrophosphoric acid (${H_4}{P_2}{O_7}$ ) respectively are:
A. Four and five
B. Four and four
C. Five and five
D. Five and four
Answer
585.3k+ views
Hint: Pyrophosphoric acid is an inorganic compound with the chemical formula ${H_4}{P_2}{O_7}$ . It is an odorless and colorless compound and is soluble in numerous solvents such as water, diethyl ether and ethyl alcohol. It is an anhydrous acid and crystallizes in two polymorphic forms.
Complete step by step answer:
The structure of pyrophosphoric acid is as follows:
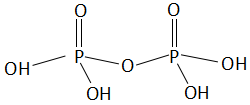
As we can clearly see in the structure, the number of $P - OH$ bonds is 4.
Now let us determine the oxidation state of phosphorus atoms in the above structure of pyrophosphoric acid. The oxygen is an electronegative element and will always carry a negative charge. Now, it depends on its surrounding atoms about what charge it carries. In case of a hydroxyl group, the hydrogen carries a +1 charge. So, the opposite charge generated on the oxygen atom along with magnitude will be -1. In the case of only oxygen atoms, we know that the oxidation number of oxygen is equal to -2. So, the charge generated on the adjacent atom of oxygen will be +2. Thus, the oxidation state of phosphorus atom will be generated because of:
Oxidation state on phosphorous = $[2 \times (P - OH) + (1 \times = O) + (1 \times - O)]$ oxidation states
Thus, the oxidation state of both the phosphorus atoms will be:
${P_{O.S}} = 2 \times ( + 1) + 1 \times ( + 2) + 1 \times ( + 1) = + 5$
Thus, the correct option is A. Four and five.
Note:
Pyrophosphoric acid acts as a source of producing phosphoric acid. Since it is an anhydrous acid, on hydrolysis of one mole of pyrophosphoric acid, two moles of phosphoric acid is obtained. The reaction takes place as follows:
${H_4}{P_2}{O_7} + {H_2}O \to 2{H_3}P{O_4}$
Complete step by step answer:
The structure of pyrophosphoric acid is as follows:

As we can clearly see in the structure, the number of $P - OH$ bonds is 4.
Now let us determine the oxidation state of phosphorus atoms in the above structure of pyrophosphoric acid. The oxygen is an electronegative element and will always carry a negative charge. Now, it depends on its surrounding atoms about what charge it carries. In case of a hydroxyl group, the hydrogen carries a +1 charge. So, the opposite charge generated on the oxygen atom along with magnitude will be -1. In the case of only oxygen atoms, we know that the oxidation number of oxygen is equal to -2. So, the charge generated on the adjacent atom of oxygen will be +2. Thus, the oxidation state of phosphorus atom will be generated because of:
Oxidation state on phosphorous = $[2 \times (P - OH) + (1 \times = O) + (1 \times - O)]$ oxidation states
Thus, the oxidation state of both the phosphorus atoms will be:
${P_{O.S}} = 2 \times ( + 1) + 1 \times ( + 2) + 1 \times ( + 1) = + 5$
Thus, the correct option is A. Four and five.
Note:
Pyrophosphoric acid acts as a source of producing phosphoric acid. Since it is an anhydrous acid, on hydrolysis of one mole of pyrophosphoric acid, two moles of phosphoric acid is obtained. The reaction takes place as follows:
${H_4}{P_2}{O_7} + {H_2}O \to 2{H_3}P{O_4}$
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




