
The number of moles of $BaC{O_3}$, which contains $6$ mol of oxygen (atom), is:
Answer
524.7k+ views
Hint: $BaC{O_3}$ is the chemical formula of Barium carbonate which is an inorganic compound. It is poorly soluble in water and like other alkali metals, it is a white salt. $BaC{O_3}$ is mainly used to remove sulfate impurities from feedstock of the chlor-alkali process. It is also used in ceramic industries and also acts as a flux, a matting and crystallizing agent and combines with certain coloring oxides to produce colors which are not easily attainable.
Complete step by step answer:
1 mol of $BaC{O_3}$ contains 3 moles of oxygen. So we can find out the number of moles of $BaC{O_3}$ present in $6$ moles of oxygen just by dividing the number of moles of oxygen total number of oxygen moles present in one mole of $BaC{O_3}$.
$1$ Mol of $BaC{O_3}$ contains $3$ moles of oxygen
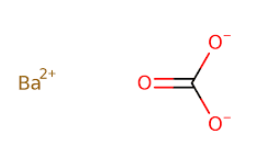
Fig: Chemical structure of $BaC{O_3}$.
$\therefore 6$ moles of oxygen present in $1$ mol of $BaC{O_3}$$ = \dfrac{6}{3} = 2$.
Therefore, the number of moles of $BaC{O_3}$ is $2$.
Note:
The above formula can also be used in finding the relation between the number of moles and molar mass.
Number of moles$ = $mass$/$relative formula mass.
This can also be used in finding the molar mass if the mass and number of moles are known.
Complete step by step answer:
1 mol of $BaC{O_3}$ contains 3 moles of oxygen. So we can find out the number of moles of $BaC{O_3}$ present in $6$ moles of oxygen just by dividing the number of moles of oxygen total number of oxygen moles present in one mole of $BaC{O_3}$.
$1$ Mol of $BaC{O_3}$ contains $3$ moles of oxygen

Fig: Chemical structure of $BaC{O_3}$.
$\therefore 6$ moles of oxygen present in $1$ mol of $BaC{O_3}$$ = \dfrac{6}{3} = 2$.
Therefore, the number of moles of $BaC{O_3}$ is $2$.
Note:
The above formula can also be used in finding the relation between the number of moles and molar mass.
Number of moles$ = $mass$/$relative formula mass.
This can also be used in finding the molar mass if the mass and number of moles are known.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




