
The number of double bonds present in the Lewis structure of $ {C_3}^{ - 4} $ is
Answer
512.4k+ views
Hint :Draw the skeletal structure of the given compound first and then write the lone pairs around the atoms to satisfy their octet. In the given molecule $ 3 $ carbons are bonded together with two double bonds and there are $ 4 $ negative charges present. The bonds between them are covalent.
Complete Step By Step Answer:
Lewis structures are the simple representations of the valence shell electrons in a molecule. It is used to show the arrangement of electrons around individual atoms in a molecule. Structures are shown by drawing dots representing electrons or lines representing bonds between the two atoms.
Let us see how these structures are drawn:
First, count the total number of valence electrons in an atom. For anions, one electron will be subtracted for every negative charge. In the case of cations, one electron will be added for every positive charge.
In the second step, the skeletal structure of the molecule or ion will be drawn, and arrange the surrounding atoms around the central atom. Each atom will be connected to the central atom with a single bond first.
The third step, write the remaining electrons as lone pairs on the terminal atoms. The remaining electrons will be such that the octet of each atom is completed.
Place all remaining electrons on the central atom, and finally,if there are not enough electrons then form multiple bonds. and thus your Lewis structure is ready.
Now using the rules let us draw the Lewis dot structure of $ {C_3}^{ - 4} $ .
It seems that there are three $ C $ atoms bonded to each other, and there are $ 4 $ negative charges on them so the Lewis structure of $ {C_3}^{ - 4} $ will be as follows:
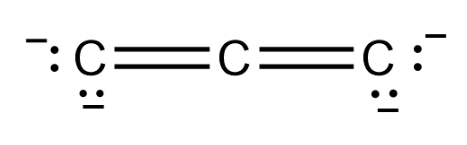
Therefore there are two double bonds in $ {C_3}^{ - 4} $
Note :
It is important to write formal charges as Lewis dot structures are incomplete without the formal charges. Always remember to add lone pairs and check whether the octet rule of every atom is satisfied. If two formal charges $ - 1 $ and $ + 1 $ are together then you can combine them to form a single lone pair.
Complete Step By Step Answer:
Lewis structures are the simple representations of the valence shell electrons in a molecule. It is used to show the arrangement of electrons around individual atoms in a molecule. Structures are shown by drawing dots representing electrons or lines representing bonds between the two atoms.
Let us see how these structures are drawn:
First, count the total number of valence electrons in an atom. For anions, one electron will be subtracted for every negative charge. In the case of cations, one electron will be added for every positive charge.
In the second step, the skeletal structure of the molecule or ion will be drawn, and arrange the surrounding atoms around the central atom. Each atom will be connected to the central atom with a single bond first.
The third step, write the remaining electrons as lone pairs on the terminal atoms. The remaining electrons will be such that the octet of each atom is completed.
Place all remaining electrons on the central atom, and finally,if there are not enough electrons then form multiple bonds. and thus your Lewis structure is ready.
Now using the rules let us draw the Lewis dot structure of $ {C_3}^{ - 4} $ .
It seems that there are three $ C $ atoms bonded to each other, and there are $ 4 $ negative charges on them so the Lewis structure of $ {C_3}^{ - 4} $ will be as follows:
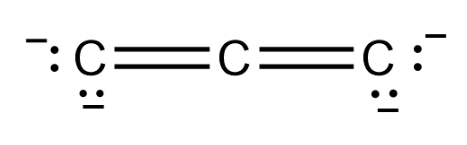
Therefore there are two double bonds in $ {C_3}^{ - 4} $
Note :
It is important to write formal charges as Lewis dot structures are incomplete without the formal charges. Always remember to add lone pairs and check whether the octet rule of every atom is satisfied. If two formal charges $ - 1 $ and $ + 1 $ are together then you can combine them to form a single lone pair.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




