
The number of atoms in the hcp unit cell is ……..
Answer
525.6k+ views
Hint: The hcp is referred to as Hexagonal Closest Packing. It consists of three layers of atoms. The hcp structure is a common structure for the elemental metals such as magnesium, zinc, beryllium, zinc, cadmium and zirconium.
Complete step-by-step answer:
The arrangement in hcp follows the order ABAB. The structure of hcp is given below.
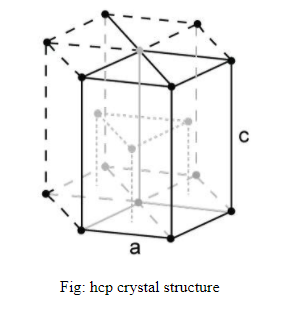
We can calculate the number of atoms in hcp unit cell as follows:
1. In HCP, there are 6 corner atoms in the top layer and 6 corner atoms in the bottom layer, so 12 atoms in the unit cell. The contribution of each atom in the unit cell is one-sixth.
2. 2 atoms are present at two face centres. The contribution of each atom in the unit cell is one-half.
3. 3 atoms are placed within the volume of the unit cell and contribution of each atom is 1.
Adding all these:
The number of atoms in hcp unit cell is $ = 12 \times \dfrac{1}{6} + 2 \times \dfrac{1}{2} + 3 = 6$
Therefore, the number of atoms in the hcp unit cell is 6.
Note: Students may get confused between the various crystal systems. The hexagonal close packing is shortly written as hcp. The hcp structure is a right rhombic prism unit cell. It has two equal edges say a and height say c which is perpendicular to the two base axes and has an angle of $120^\circ $.
Complete step-by-step answer:
The arrangement in hcp follows the order ABAB. The structure of hcp is given below.

We can calculate the number of atoms in hcp unit cell as follows:
1. In HCP, there are 6 corner atoms in the top layer and 6 corner atoms in the bottom layer, so 12 atoms in the unit cell. The contribution of each atom in the unit cell is one-sixth.
2. 2 atoms are present at two face centres. The contribution of each atom in the unit cell is one-half.
3. 3 atoms are placed within the volume of the unit cell and contribution of each atom is 1.
Adding all these:
The number of atoms in hcp unit cell is $ = 12 \times \dfrac{1}{6} + 2 \times \dfrac{1}{2} + 3 = 6$
Therefore, the number of atoms in the hcp unit cell is 6.
Note: Students may get confused between the various crystal systems. The hexagonal close packing is shortly written as hcp. The hcp structure is a right rhombic prism unit cell. It has two equal edges say a and height say c which is perpendicular to the two base axes and has an angle of $120^\circ $.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




