
The non-aromatic compound among the following is:-
A
B
C
D




Answer
587.1k+ views
Hint:Non-aromatic compounds are cyclic compounds which do not have a closed loop of p-orbitals that is because it is not conjugation of $\pi $ (pi) – electrons. Also, the non-aromatic compounds are non- planar ring systems.
Complete step by step answer:
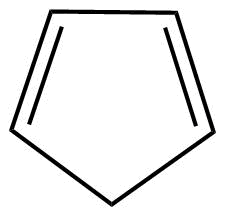
A: As we know that cyclopentadiene (${C_5}{H_6}$) is not having conjugation so definitely it is not an aromatic compound. Also, the huckel's rule (4n+2) electrons conjugation which is applicable for aromatic compounds, is not being followed here.
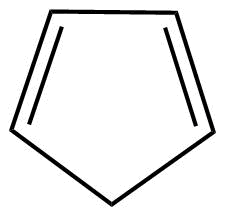
Hence, we can say that this is a non-aromatic compound.
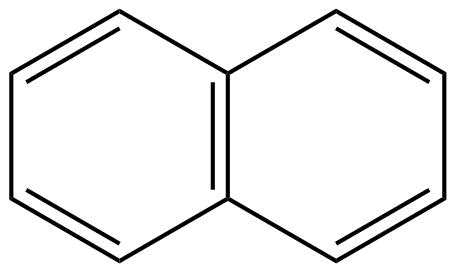
B: Naphthalene is an aromatic compound because it shows the continuous delocalisation of pi ($\pi $) – electrons that are naphthalene have conjugation.
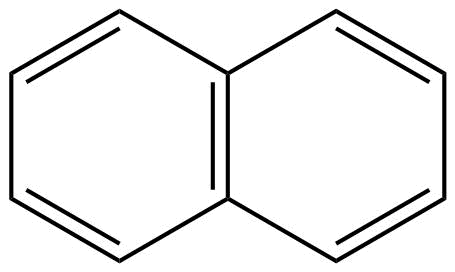
Moreover, naphthalene is also having a planar structure. Hence, we can say that naphthalene is an aromatic compound.
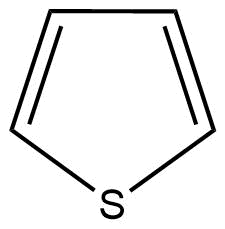
C: Thiophene is a heterocyclic compound with the chemical formula ${C_4}{H_4}S$. It is a planar five-membered ring.
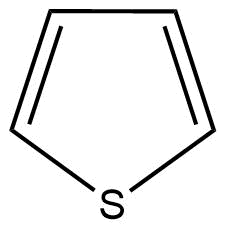
Here, the conjugation of delocalised $\pi $ (pi) electrons can be also seen. Hence, thiophene is also an aromatic compound.
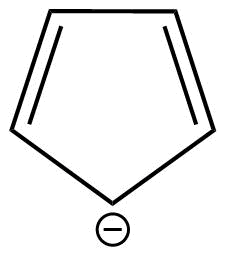
D: Cyclopentadienide is a monocyclic aromatic compound.
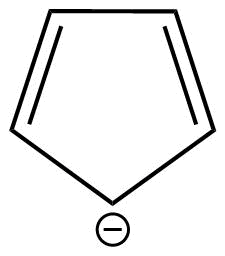
This is because cyclopentadienide shows conjugation and huckel's rule is also applicable in case of this compound.
So, the correct option is (A).
Note:
The non-aromatic compounds without a ring structure are termed without a ring structure are termed as aliphatic whereas those with a cyclic structure are called alicyclic.
Complete step by step answer:
A: As we know that cyclopentadiene (${C_5}{H_6}$) is not having conjugation so definitely it is not an aromatic compound. Also, the huckel's rule (4n+2) electrons conjugation which is applicable for aromatic compounds, is not being followed here.

Hence, we can say that this is a non-aromatic compound.
B: Naphthalene is an aromatic compound because it shows the continuous delocalisation of pi ($\pi $) – electrons that are naphthalene have conjugation.

Moreover, naphthalene is also having a planar structure. Hence, we can say that naphthalene is an aromatic compound.
C: Thiophene is a heterocyclic compound with the chemical formula ${C_4}{H_4}S$. It is a planar five-membered ring.

Here, the conjugation of delocalised $\pi $ (pi) electrons can be also seen. Hence, thiophene is also an aromatic compound.
D: Cyclopentadienide is a monocyclic aromatic compound.

This is because cyclopentadienide shows conjugation and huckel's rule is also applicable in case of this compound.
So, the correct option is (A).
Note:
The non-aromatic compounds without a ring structure are termed without a ring structure are termed as aliphatic whereas those with a cyclic structure are called alicyclic.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




