
The nature of 2,4,6-trinitrophenol is
A. neutral
B. basic
C. acidic
D. weak basic
Answer
577.2k+ views
Hint:. This compound is also known as Picric Acid. There is an effect of electron withdrawing as well as electron donating groups on Phenol.
The acidity of the Phenol is increased when there are electron withdrawing groups are attached and decreases when there are donating groups attached to the compound.
Complete step by step answer:
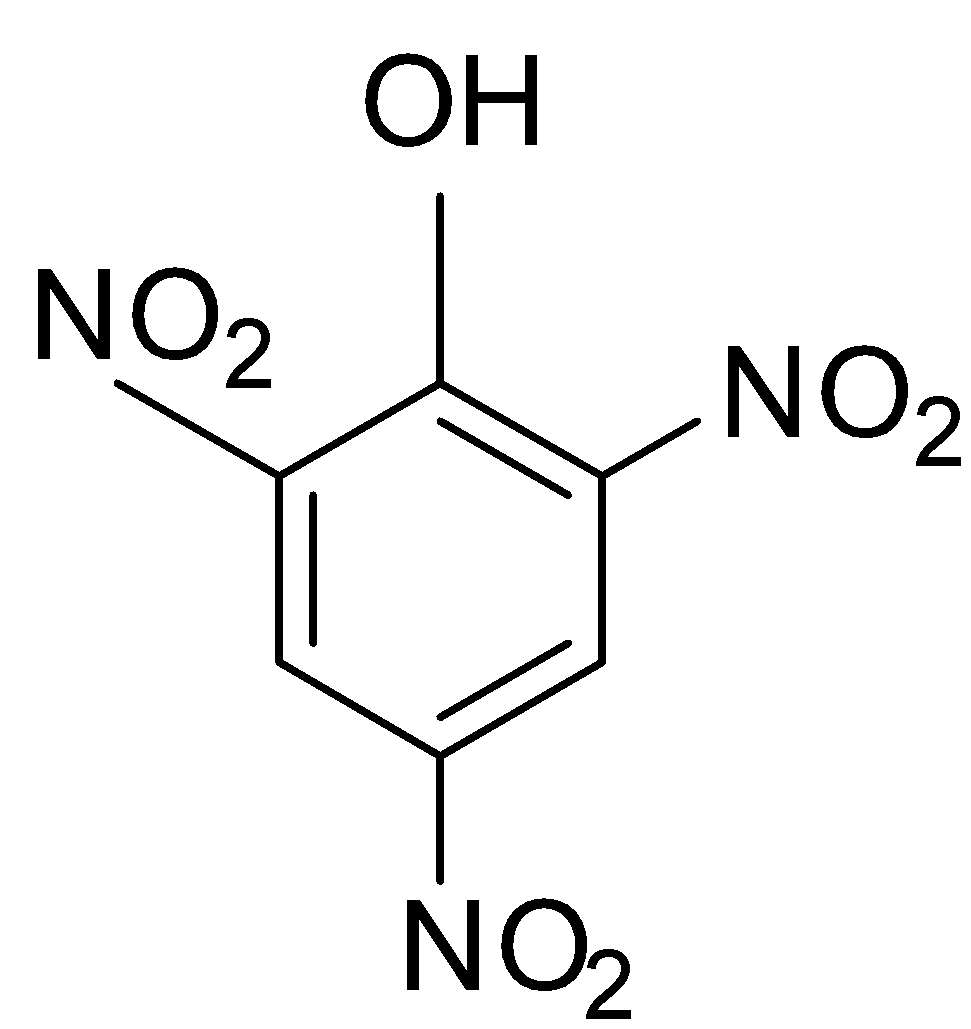
If we look at the structure of the picric acid or 2,4,6-trinitrophenol, we see that there are three nitro groups attached at the 2nd, 4th and 6th position of the compound.
The nitro group is an electron withdrawing and deactivating group and they are attached to the ortho and para position. Hence, during the resonance stabilisation, they withdraw electrons from the ring due to which there is deficiency of electrons in the ring system, leading to the destabilisation.
Due to which the OH group being an activator and electron donor, undergoes resonance and there is a partial-positive charge induced on the Oxygen which is against the nature of the Oxygen and the bond of the O-H becomes weaker, ultimately leading to the bond dissociation. These observations suggest that 2,4,6-trinitrophenol is acidic in nature.
So, the correct answer is “Option C”.
Note: The acidity of nitro-phenols varies in the order meta < ortho < para, hence, para nitro-phenols are more acidic. The Nitro group attached to a conjugated system exhibits strong -M effect which decreases electron density at ortho and para positions than at meta position. Hence , the \[p{{K}_{a}}\] of picric acid 0.38, even lesser than carboxylic acids.
Smaller the \[p{{K}_{a}}\], stronger the acid.
The acidity of the Phenol is increased when there are electron withdrawing groups are attached and decreases when there are donating groups attached to the compound.
Complete step by step answer:

If we look at the structure of the picric acid or 2,4,6-trinitrophenol, we see that there are three nitro groups attached at the 2nd, 4th and 6th position of the compound.
The nitro group is an electron withdrawing and deactivating group and they are attached to the ortho and para position. Hence, during the resonance stabilisation, they withdraw electrons from the ring due to which there is deficiency of electrons in the ring system, leading to the destabilisation.
Due to which the OH group being an activator and electron donor, undergoes resonance and there is a partial-positive charge induced on the Oxygen which is against the nature of the Oxygen and the bond of the O-H becomes weaker, ultimately leading to the bond dissociation. These observations suggest that 2,4,6-trinitrophenol is acidic in nature.
So, the correct answer is “Option C”.
Note: The acidity of nitro-phenols varies in the order meta < ortho < para, hence, para nitro-phenols are more acidic. The Nitro group attached to a conjugated system exhibits strong -M effect which decreases electron density at ortho and para positions than at meta position. Hence , the \[p{{K}_{a}}\] of picric acid 0.38, even lesser than carboxylic acids.
Smaller the \[p{{K}_{a}}\], stronger the acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




