
The monomers used in the preparation of Dextron are:
(A) Lactic acid and glycolic acid
(B) 3-hydroxy butanoic acid and 3-hydroxy pentanoic acid
(C) Styrene and 1,3-butadiene
(D) Hexamethylenediamine and adipic acid
Answer
594.3k+ views
Hint: Dextron is a type of a polyester copolymer. Polyester polymers are generally prepared by condensation polymerization in which generally carboxylic acid functional groups react with hydroxyl groups to remove water molecules.
Complete answer: You need to know the structure of the polymer in order to identify the monomers used in its preparation. Dextron is a copolymer and it is made by polymerization of two monomers. The structure of the Dextron polymer is given below.
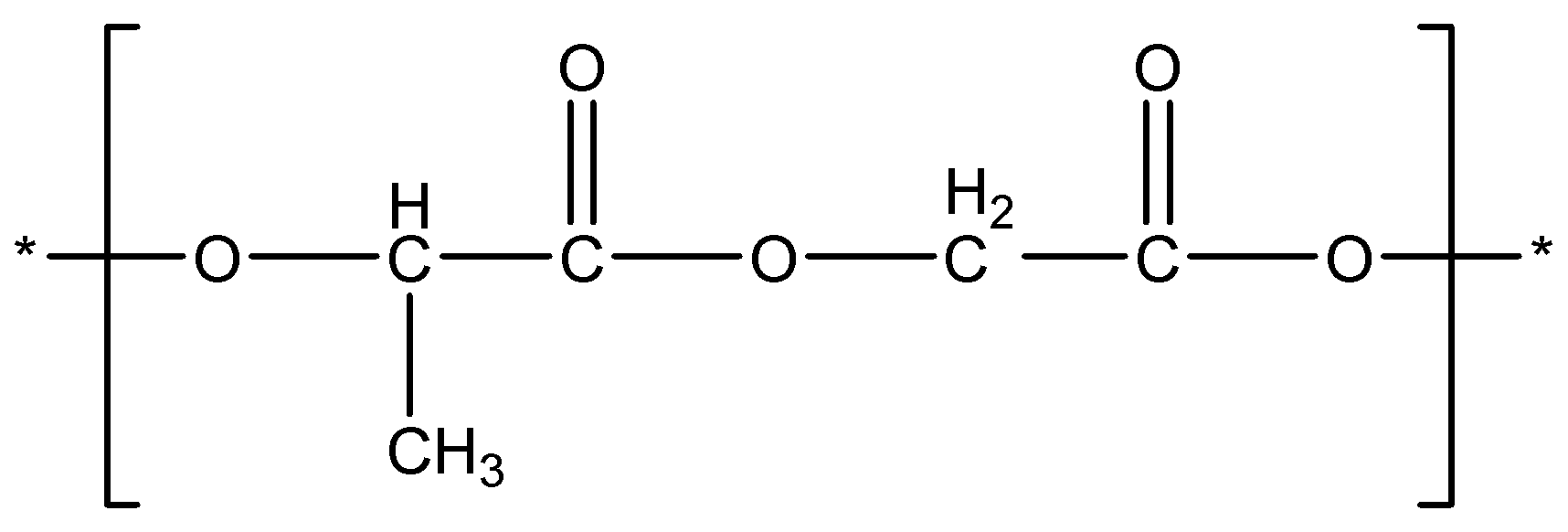
Let’s compare it with the monomers given in the options.
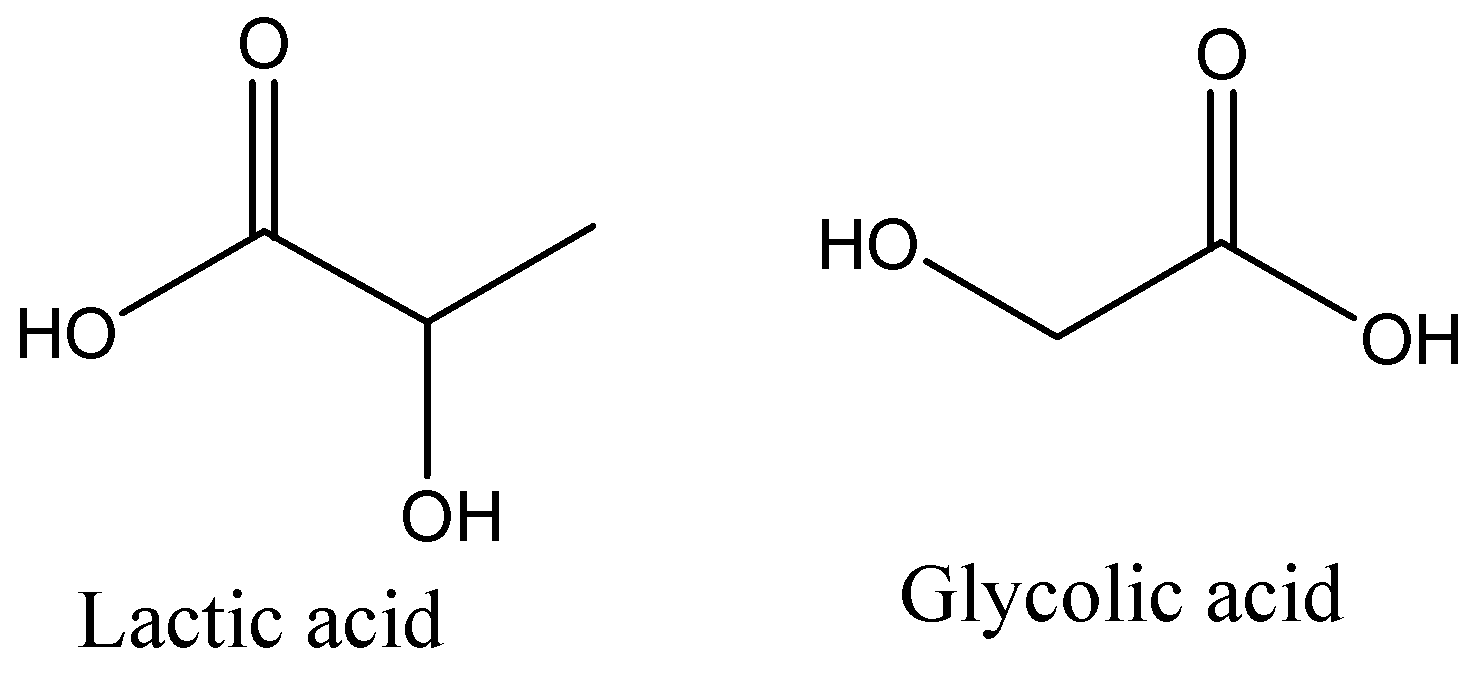
- We can see that Lactic acid is having 3 carbons and Glycolic acid is having 2 carbon in their structures and we can also see that the polymer unit is having 5 carbons in total. So, it is possible that it may be a polymer of that monomer. If hydroxyl group at carbon-2 in glycolic acid reacts with -COOH group of Lactic acid to lose water, and the same way carbon-2 of Lactic acid reacts with -COOH group of glycolic acid to lose water then this copolymer will be formed. So, it is clear that Lactic acid and Glycolic acid polymerise to give Dexron. Let’s review other options also.
- 3-hydroxy butanoic acid and 3-hydroxy pentanoic acid polymerize to give polymer PHBV and it has 9 carbon in its polymer unit.
- The polymer that is formed by polymerization of styrene and 1,3-butadiene is named as styrene-butadiene copolymer.
- Hexamethylenediamine and adipic acid combine as monomer units in the polymerization process to give Nylon-6,6 polymer.
So, correct answer for this question is (A) Lactic acid and glycolic acid
So, the correct answer is “Option A”.
Note: Do not get confused between Dextron, Dextrin and Dextran. Dextran is a polysaccharide derived from glucose and Dextrin is also a kind of carbohydrate. Remember that in this condensation reaction, two hydroxyl groups cannot react to remove water molecules.
Complete answer: You need to know the structure of the polymer in order to identify the monomers used in its preparation. Dextron is a copolymer and it is made by polymerization of two monomers. The structure of the Dextron polymer is given below.

Let’s compare it with the monomers given in the options.

- We can see that Lactic acid is having 3 carbons and Glycolic acid is having 2 carbon in their structures and we can also see that the polymer unit is having 5 carbons in total. So, it is possible that it may be a polymer of that monomer. If hydroxyl group at carbon-2 in glycolic acid reacts with -COOH group of Lactic acid to lose water, and the same way carbon-2 of Lactic acid reacts with -COOH group of glycolic acid to lose water then this copolymer will be formed. So, it is clear that Lactic acid and Glycolic acid polymerise to give Dexron. Let’s review other options also.
- 3-hydroxy butanoic acid and 3-hydroxy pentanoic acid polymerize to give polymer PHBV and it has 9 carbon in its polymer unit.
- The polymer that is formed by polymerization of styrene and 1,3-butadiene is named as styrene-butadiene copolymer.
- Hexamethylenediamine and adipic acid combine as monomer units in the polymerization process to give Nylon-6,6 polymer.
So, correct answer for this question is (A) Lactic acid and glycolic acid
So, the correct answer is “Option A”.
Note: Do not get confused between Dextron, Dextrin and Dextran. Dextran is a polysaccharide derived from glucose and Dextrin is also a kind of carbohydrate. Remember that in this condensation reaction, two hydroxyl groups cannot react to remove water molecules.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




