
The monomer styrene has a structural formula ________.
(A) \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}} - {\text{CH}} = {\text{C}}{{\text{H}}_2}\]
(B) ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}} - {\text{C}}{{\text{H}}_2} = {\text{C}}{{\text{H}}_2}$
(C) ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{6}}} - {\text{CH}} = {\text{C}}{{\text{H}}_2}$
(D) ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{2}}} - {\text{C}}{{\text{H}}_2} = {\text{C}}{{\text{H}}_2}$
Answer
569.4k+ views
Hint:The molecule that bonds to two other identical molecules to form a polymer is known as a monomer. The monomer is the repeating unit of the polymer. Most common example of a polymer is polythene(polyethylene), which is a polymer of ethene.
Complete answer:
Styrene is an organic compound and appears as a colourless and oily liquid. Styrene can evaporate easily and has a sweet smell. Styrene occurs in plants and foods like cinnamon, coffee beans, balsam trees and peanuts. It is also found in coal tar. Styrene is flammable in nature. Long exposure to styrene can cause cancer in human beings. Other names of styrene are ethylbenzene, vinylbenzene, phenylethene, phenylethylene, cinnamene, styrol, styrolene and styropor.
The molecular formula for styrene is ${{\text{C}}_{\text{8}}}{{\text{H}}_{\text{8}}}$.
Styrene has a vinyl group $\left( { - {\text{CH}} = {\text{C}}{{\text{H}}_2}} \right)$ present in it. The presence of vinyl groups allows styrene to polymerize. Thus, styrene is a monomer.
The structure of styrene is as follows:
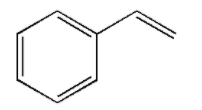
The polymerised products of styrene are polystyrene, styrene-butadiene, rubber, styrene-butadiene latex, etc. all these materials are used in manufacturing rubber, plastic, insulation, etc.
Styrene is prepared industrially by dehydrogenation of ethylbenzene, oxygenation of ethylbenzene, pyrolysis of gasoline, from the reaction of toluene and methanol, from the reaction of benzene and ethane, etc. Laboratory synthesis of styrene is done by decarboxylation of cinnamic acid.
The structural formula for monomer styrene is \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}} - {\text{CH}} = {\text{C}}{{\text{H}}_2}\].
Thus, the correct option is (A) \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}} - {\text{CH}} = {\text{C}}{{\text{H}}_2}\].
Note:
Styrene polymerizes spontaneously and does not need any external initiator. Thus, styrene is an autopolymeriser. Thus, there is a risk of thermal runaway and explosion. Styrene is a ‘known carcinogen’. Styrene affects vision and hearing functions.
Complete answer:
Styrene is an organic compound and appears as a colourless and oily liquid. Styrene can evaporate easily and has a sweet smell. Styrene occurs in plants and foods like cinnamon, coffee beans, balsam trees and peanuts. It is also found in coal tar. Styrene is flammable in nature. Long exposure to styrene can cause cancer in human beings. Other names of styrene are ethylbenzene, vinylbenzene, phenylethene, phenylethylene, cinnamene, styrol, styrolene and styropor.
The molecular formula for styrene is ${{\text{C}}_{\text{8}}}{{\text{H}}_{\text{8}}}$.
Styrene has a vinyl group $\left( { - {\text{CH}} = {\text{C}}{{\text{H}}_2}} \right)$ present in it. The presence of vinyl groups allows styrene to polymerize. Thus, styrene is a monomer.
The structure of styrene is as follows:

The polymerised products of styrene are polystyrene, styrene-butadiene, rubber, styrene-butadiene latex, etc. all these materials are used in manufacturing rubber, plastic, insulation, etc.
Styrene is prepared industrially by dehydrogenation of ethylbenzene, oxygenation of ethylbenzene, pyrolysis of gasoline, from the reaction of toluene and methanol, from the reaction of benzene and ethane, etc. Laboratory synthesis of styrene is done by decarboxylation of cinnamic acid.
The structural formula for monomer styrene is \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}} - {\text{CH}} = {\text{C}}{{\text{H}}_2}\].
Thus, the correct option is (A) \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}} - {\text{CH}} = {\text{C}}{{\text{H}}_2}\].
Note:
Styrene polymerizes spontaneously and does not need any external initiator. Thus, styrene is an autopolymeriser. Thus, there is a risk of thermal runaway and explosion. Styrene is a ‘known carcinogen’. Styrene affects vision and hearing functions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




