
The monomer of Biodegradable polymer, nylon 2-nylon 6 are:
(A) glycine + adipic acid
(B) glycol + phthalic acid
(C) phenol + Urea
(D) glycine + amino caproic acid:
Answer
578.7k+ views
Hint: Biodegradable polymer is those polymers which can be broken down into some form of biomass, inorganic salts, water, carbon dioxide and nitrogen by the action of bacteria. Biodegradable polymer is non-toxic, it does not harm us in any way.
Complete answer:
First, what is a biodegradable polymer? When a polymer can be broken down into natural by-products such as carbon dioxide, nitrogen, water, inorganic salt and biomass by bacterial decomposition. Such polymers are called Biodegradable polymers. Some examples of biodegradable polymers are Dextron, PHBV (Polyhydroxy butyrates-co-beta hydroxyvalerate), Nylon 2-nylon 6.
Nylon 2-nylon 6 can be formed by polyamide copolymerization. This reaction takes place between two monomers namely Glycine and amino caproic acid. The formula for glycine is \[N{H_2}C{H_2}COOH\] and the formula for amino caproic acid is \[N{H_2}{(C{H_2})_5}COOH\].
This a type of condensation reaction in which when n moles of glycine, \[N{H_2}C{H_2}COOH\] and n moles of amino caproic acid, \[N{H_2}{(C{H_2})_5}COOH\] will react and further removal of water molecules takes place giving rise to the polymer, Nylon 2-Nylon 6. The reaction is given below:
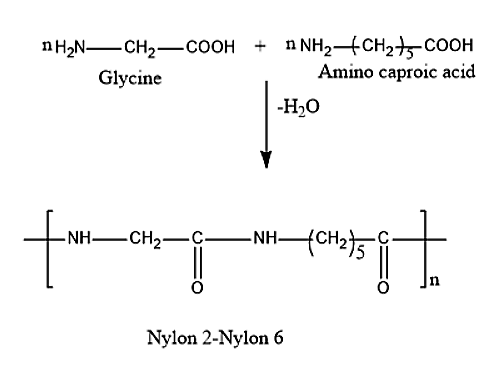
The monomers of Biodegradable polymer, nylon 2-nylon 6 are glycine and amino caproic acid.
Therefore, the correct answer is option(D) glycine + amino caproic acid.
Additional information:
The characteristics of biodegradable polymers are given below.
- Biodegradable polymers have good bio-compatibility.
- They are highly nontoxic.
- It is environmentally friendly.
- It is low cost.
- It is capable of controlling the rate of degradation.
Note: Nylon 2-Nylon 6 have various uses such as
- It can be used in the synthesis of Artificial fibers.
- Strings of musical instruments are made using Nylon 2-Nylon 6.
- Bristles of toothbrushes are made from this.
Complete answer:
First, what is a biodegradable polymer? When a polymer can be broken down into natural by-products such as carbon dioxide, nitrogen, water, inorganic salt and biomass by bacterial decomposition. Such polymers are called Biodegradable polymers. Some examples of biodegradable polymers are Dextron, PHBV (Polyhydroxy butyrates-co-beta hydroxyvalerate), Nylon 2-nylon 6.
Nylon 2-nylon 6 can be formed by polyamide copolymerization. This reaction takes place between two monomers namely Glycine and amino caproic acid. The formula for glycine is \[N{H_2}C{H_2}COOH\] and the formula for amino caproic acid is \[N{H_2}{(C{H_2})_5}COOH\].
This a type of condensation reaction in which when n moles of glycine, \[N{H_2}C{H_2}COOH\] and n moles of amino caproic acid, \[N{H_2}{(C{H_2})_5}COOH\] will react and further removal of water molecules takes place giving rise to the polymer, Nylon 2-Nylon 6. The reaction is given below:

The monomers of Biodegradable polymer, nylon 2-nylon 6 are glycine and amino caproic acid.
Therefore, the correct answer is option(D) glycine + amino caproic acid.
Additional information:
The characteristics of biodegradable polymers are given below.
- Biodegradable polymers have good bio-compatibility.
- They are highly nontoxic.
- It is environmentally friendly.
- It is low cost.
- It is capable of controlling the rate of degradation.
Note: Nylon 2-Nylon 6 have various uses such as
- It can be used in the synthesis of Artificial fibers.
- Strings of musical instruments are made using Nylon 2-Nylon 6.
- Bristles of toothbrushes are made from this.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Organisms of a higher trophic level which feed on several class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE




