
The molecule with non-zero dipole moment?
(a)- $BC{{l}_{3}}$
(b)- $BeC{{l}_{2}}$
(c)- $CC{{l}_{4}}$
(d)- $NC{{l}_{3}}$
Answer
588.9k+ views
Hint: The dipole moment is the product of negative or positive charge and the distance between the centers of positive and negative charges. The dipole moment is directed towards the more electronegative atom in the compound. The opposite direction of the dipole moment in the molecule cancels each other.
Complete step by step answer:
The dipole moment is the product of the positive or negative charge and the distance between centers of the positive and negative charges. It is denoted by$\mu $. It is denoted by the arrow with its tail at the positive center and head pointing towards the negative end:
$(+\mapsto -)$
The dipole moment is directed towards the more electronegative atom.
Let us see all the options:
(a)- $BC{{l}_{3}}$
The structure of $BC{{l}_{3}}$is:
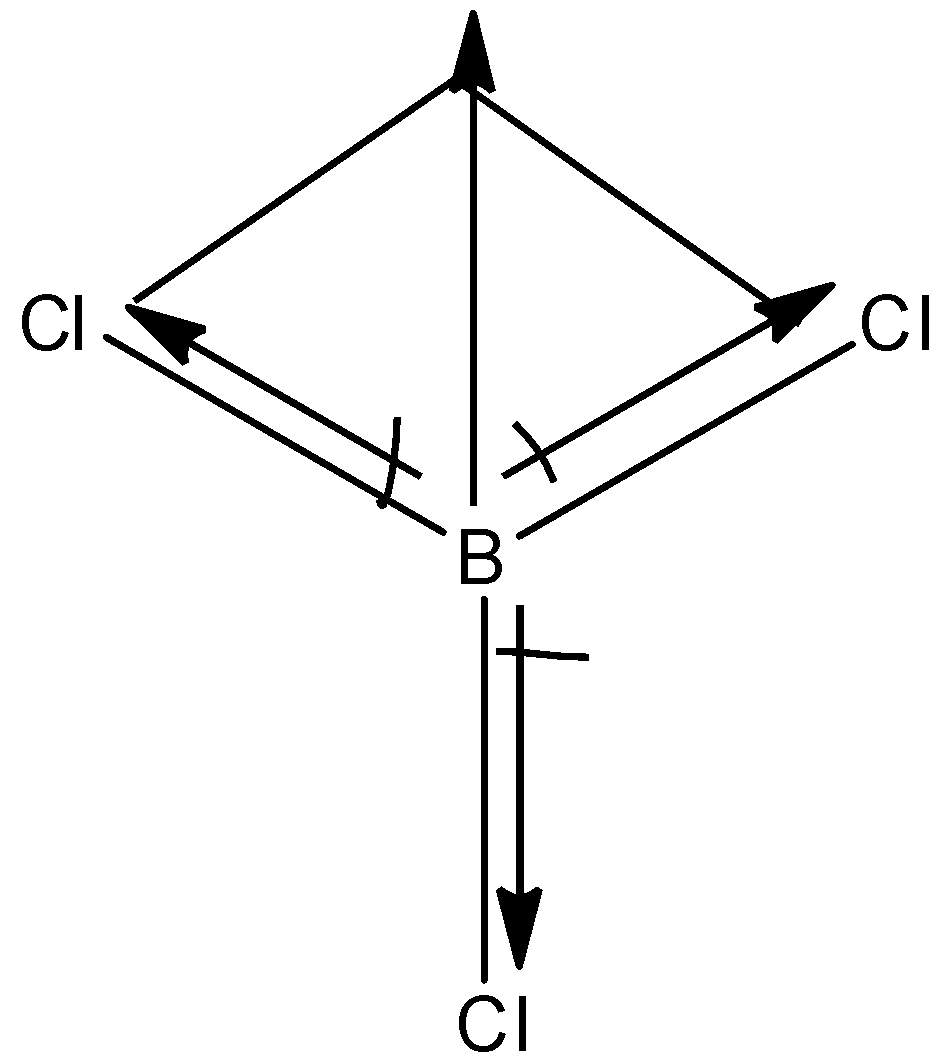
The chlorine atom is more electronegative than the boron atom hence, the dipole moment will direct towards the chlorine atom. Since the dipole moment of one chlorine atom is canceled by the resultant dipole moment of the other 2 chlorine atoms. Hence, it has zero dipole moment.
(b)- $BeC{{l}_{2}}$
The structure of $BeC{{l}_{2}}$is:

The chlorine atom is more electronegative than the beryllium atom hence, the dipole moment will direct towards the chlorine atom. Both the dipole moments of the chlorine atom are in the opposite direction and hence cancel out. So, the dipole moment is zero.
(c)- $CC{{l}_{4}}$
The structure of $CC{{l}_{4}}$is:
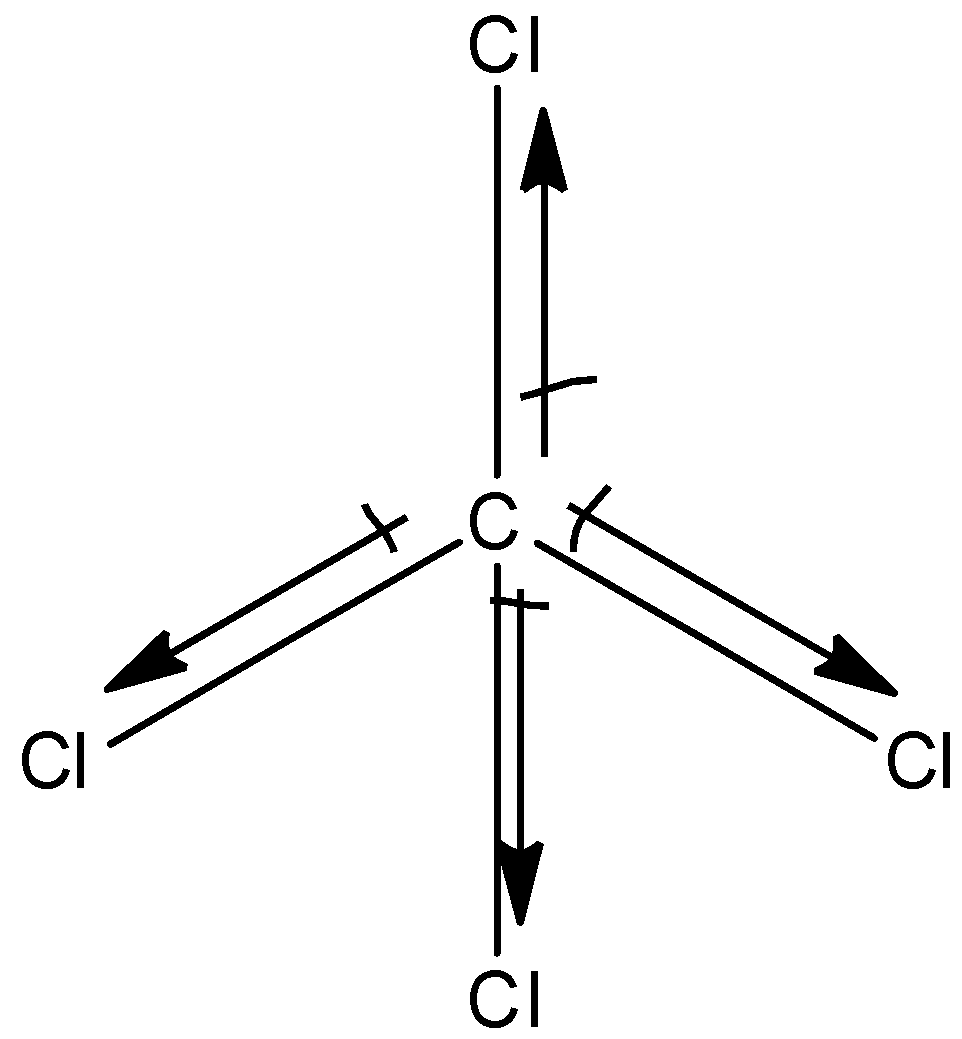
The chlorine atom is more electronegative than the carbon atom hence, the dipole moment will direct towards the chlorine atom. It is a symmetrical molecule, and the molecular dipole moment is found to be zero.
(d)- $NC{{l}_{3}}$
The structure of $NC{{l}_{3}}$is:
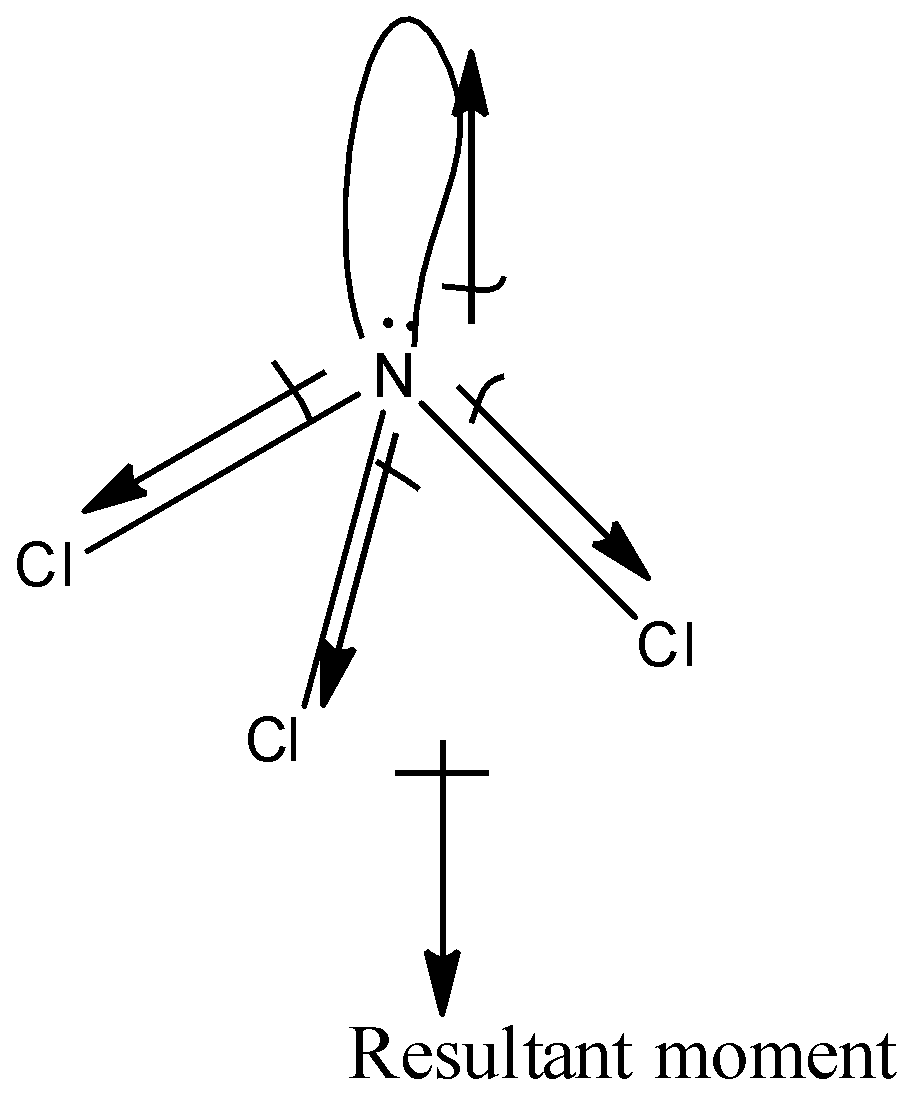
The chlorine atom is more electronegative than the nitrogen atom hence, the dipole moment will direct towards the chlorine atom. Since the $NC{{l}_{3}}$has a pyramidal structure due to the presence of a lone pair, there is a net resultant dipole moment.
Hence, the correct answer is an option (d)- $NC{{l}_{3}}$
Note: The determination of the dipole moment helps find the polarity of the molecule. The higher the dipole moment of the molecule, the higher is the polarity of the molecule. It can also be used in calculating the ionic character.
Complete step by step answer:
The dipole moment is the product of the positive or negative charge and the distance between centers of the positive and negative charges. It is denoted by$\mu $. It is denoted by the arrow with its tail at the positive center and head pointing towards the negative end:
$(+\mapsto -)$
The dipole moment is directed towards the more electronegative atom.
Let us see all the options:
(a)- $BC{{l}_{3}}$
The structure of $BC{{l}_{3}}$is:

The chlorine atom is more electronegative than the boron atom hence, the dipole moment will direct towards the chlorine atom. Since the dipole moment of one chlorine atom is canceled by the resultant dipole moment of the other 2 chlorine atoms. Hence, it has zero dipole moment.
(b)- $BeC{{l}_{2}}$
The structure of $BeC{{l}_{2}}$is:

The chlorine atom is more electronegative than the beryllium atom hence, the dipole moment will direct towards the chlorine atom. Both the dipole moments of the chlorine atom are in the opposite direction and hence cancel out. So, the dipole moment is zero.
(c)- $CC{{l}_{4}}$
The structure of $CC{{l}_{4}}$is:

The chlorine atom is more electronegative than the carbon atom hence, the dipole moment will direct towards the chlorine atom. It is a symmetrical molecule, and the molecular dipole moment is found to be zero.
(d)- $NC{{l}_{3}}$
The structure of $NC{{l}_{3}}$is:

The chlorine atom is more electronegative than the nitrogen atom hence, the dipole moment will direct towards the chlorine atom. Since the $NC{{l}_{3}}$has a pyramidal structure due to the presence of a lone pair, there is a net resultant dipole moment.
Hence, the correct answer is an option (d)- $NC{{l}_{3}}$
Note: The determination of the dipole moment helps find the polarity of the molecule. The higher the dipole moment of the molecule, the higher is the polarity of the molecule. It can also be used in calculating the ionic character.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




