
The molecule which does not exhibit strong hydrogen bonding is:
A. Methyl amine
B. Diethyl ether
C. Acetic acid
D. Glucose
Answer
599.1k+ views
Hint: “Hydrogen bonding is a special type of dipole-dipole attraction or bond between molecules”. It is not a covalent bond. Only those compounds in which Hydrogen atom is attached directly with a highly electronegative atoms i.e. N, O or F are able to show the property called hydrogen bonding.
Complete step by step answer:
- Hydrogen bonding is a weak attractive force formed between different molecules.
- Coming to given options, Option A, Methyl amine.
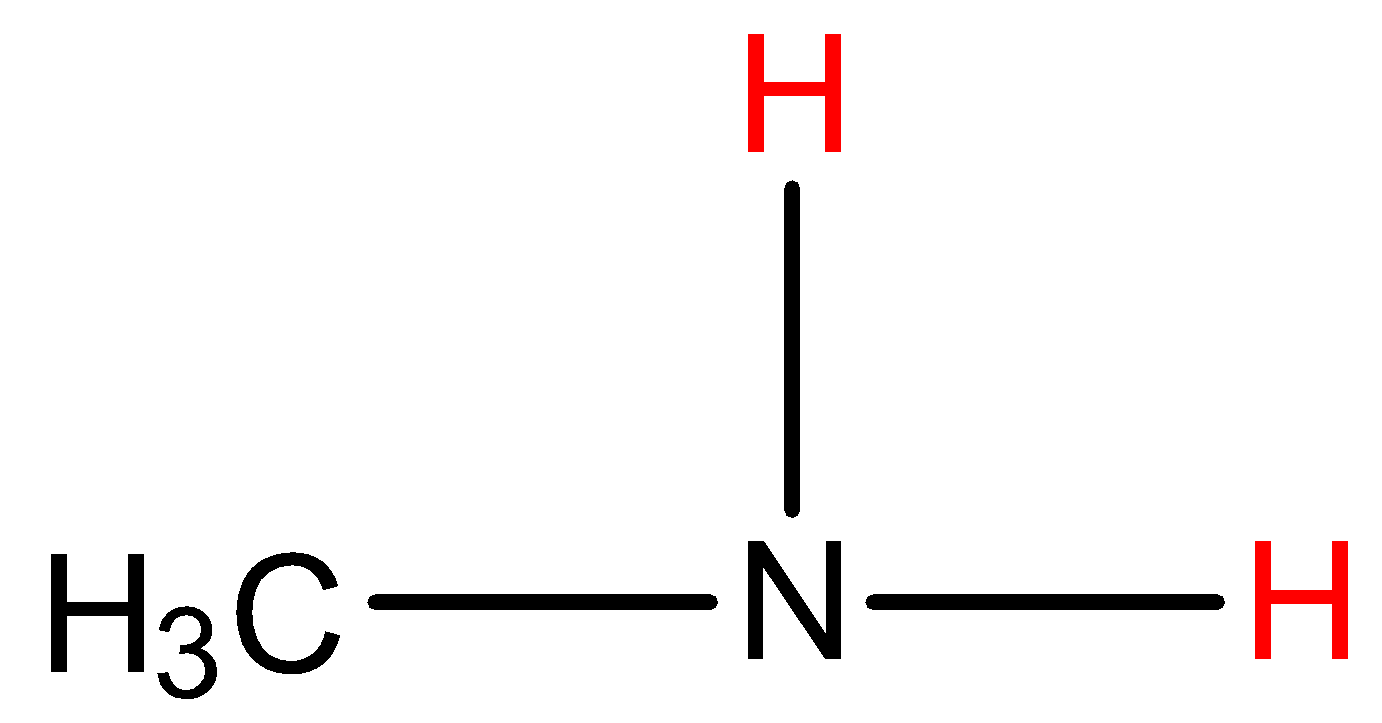
- In the structure the red marked hydrogens are directly attached with nitrogen which has high electronegativity. So, methylamine shows hydrogen bonding with other methyl amine molecules.
- Coming to option B, diethyl ether.
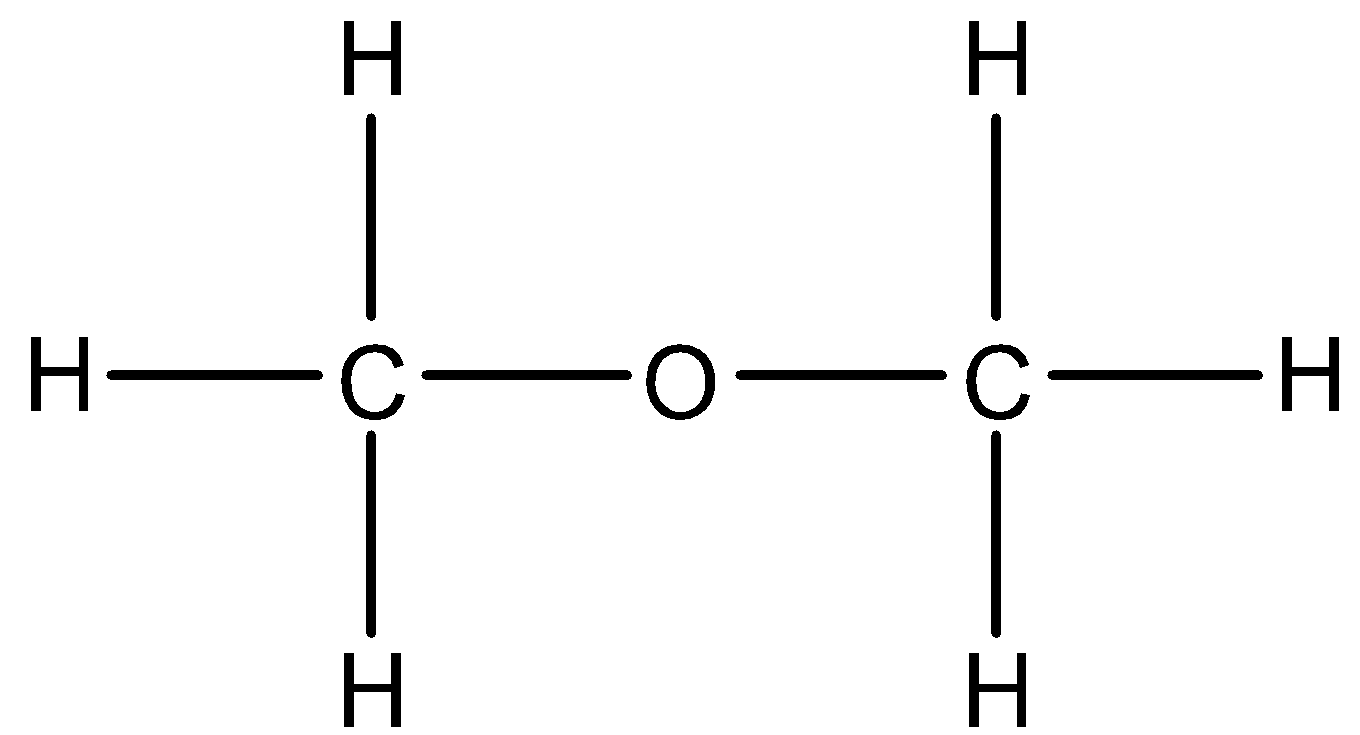
- In diethyl ether the hydrogen is not directly attached to high electronegativity atoms. So, diethyl ether does not show hydrogen bonding.
- Coming to option C, acetic acid.
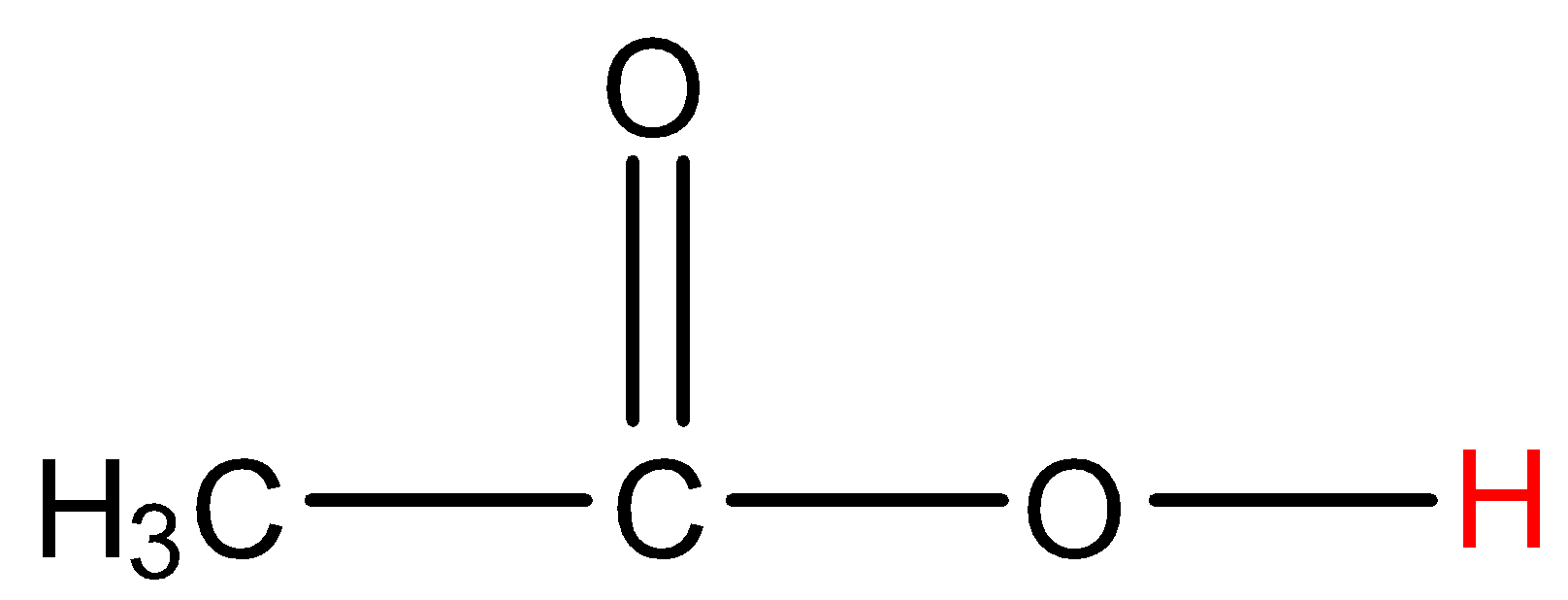
- The hydrogen which is marked with red color is directly attached to a highly electronegative atom. So, acetic acid shows hydrogen bonding.
- Coming to option D, Glucose.
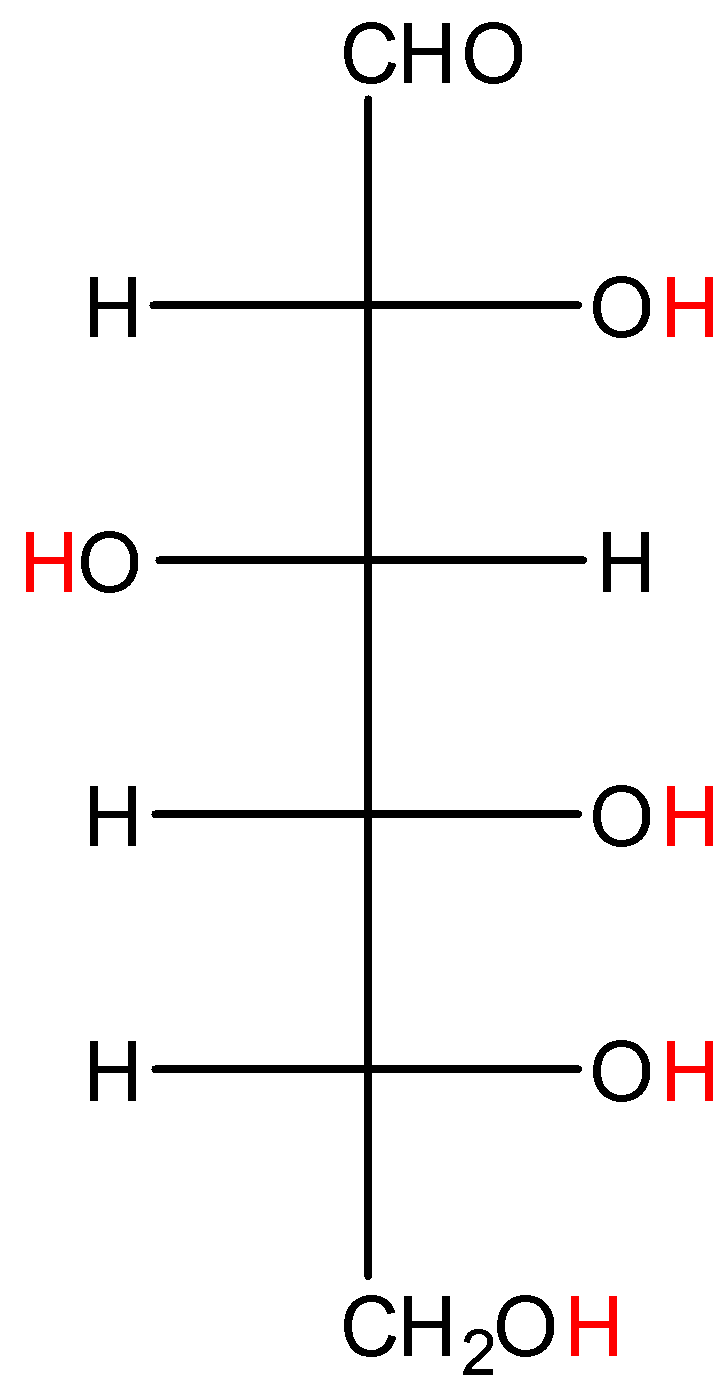
- In glucose also the hydrogens marked with red color are directly attached to high electronegativity atoms. So, glucose shows hydrogen bonding.
So, the correct option is B, diethyl ether won’t show the hydrogen bonding.
Note: Don’t be confused with the words, hydrogen bond and covalent bond. Both are different.
Covalent bond: Covalent bond is going to form between two atoms having less electronegativity difference.
Hydrogen Bond: Hydrogens which are attached to high electronegative atoms like N, O, F shows the hydrogen bonding with the other molecules.
Complete step by step answer:
- Hydrogen bonding is a weak attractive force formed between different molecules.
- Coming to given options, Option A, Methyl amine.

- In the structure the red marked hydrogens are directly attached with nitrogen which has high electronegativity. So, methylamine shows hydrogen bonding with other methyl amine molecules.
- Coming to option B, diethyl ether.

- In diethyl ether the hydrogen is not directly attached to high electronegativity atoms. So, diethyl ether does not show hydrogen bonding.
- Coming to option C, acetic acid.

- The hydrogen which is marked with red color is directly attached to a highly electronegative atom. So, acetic acid shows hydrogen bonding.
- Coming to option D, Glucose.

- In glucose also the hydrogens marked with red color are directly attached to high electronegativity atoms. So, glucose shows hydrogen bonding.
So, the correct option is B, diethyl ether won’t show the hydrogen bonding.
Note: Don’t be confused with the words, hydrogen bond and covalent bond. Both are different.
Covalent bond: Covalent bond is going to form between two atoms having less electronegativity difference.
Hydrogen Bond: Hydrogens which are attached to high electronegative atoms like N, O, F shows the hydrogen bonding with the other molecules.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




