
The molecular formula of glycerol is:
A.$\text{C}{{\text{H}}_{3}}\text{OH}$
B. ${{\text{C}}_{2}}{{\text{H}}_{6}}{{\text{O}}_{2}}$
C. ${{\text{C}}_{4}}{{\text{H}}_{10}}{{\text{O}}_{4}}$
D. ${{\text{C}}_{3}}{{\text{H}}_{8}}{{\text{O}}_{3}}$
Answer
594.9k+ views
Hint: The molecular formula is determined by the count and the type of number of atoms present in a molecule. Molecular formula is the actual count of atoms present in a molecule of each element.
Complete step by step solution:
Glycerol is a trihydric alcohol, it contains 3 $\text{C}$ (carbon), 8$\text{H}$(hydrogen), 3$\text{O}$(oxygen) atoms in it, as suggested by the structure of glycerol. It has three alcohol groups present in it.
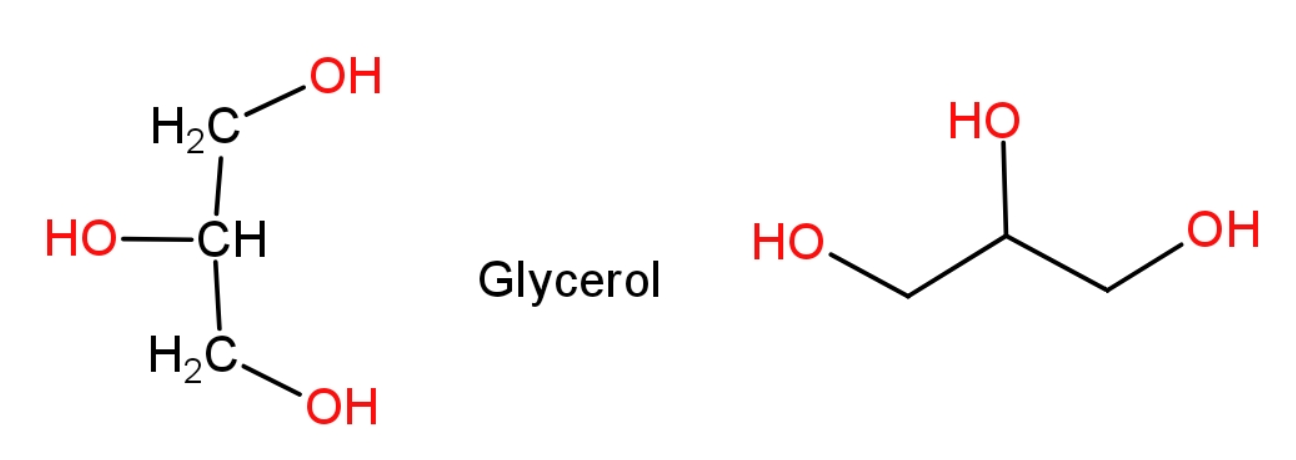
The molecular formula can be directly written as the count of atoms is always written in subscript after the symbol of that element.
Carbon is denoted by the symbol ‘$\text{C}$’.
Hydrogen is denoted by the symbol ‘$\text{H}$’.
Oxygen is denoted by the symbol ‘$\text{O}$’.
The molecular formula of glycerol becomes as we have both the elements in the structure, with their symbols and the count of elements present in the compound.
Molecular Formula:${{\text{C}}_{3}}{{\text{H}}_{8}}{{\text{O}}_{3}}$
The molecular formula of glycerol becomes${{\text{C}}_{3}}{{\text{H}}_{8}}{{\text{O}}_{3}}$, which is option ‘d’ which matches the answer completely.
A. $\text{C}{{\text{H}}_{3}}\text{OH}$ is methyl alcohol or methanol with 1 alcohol group present.
B. ${{\text{C}}_{2}}{{\text{H}}_{6}}{{\text{O}}_{2}}$ is ethylene glycol with two alcohol groups present.
C. ${{\text{C}}_{4}}{{\text{H}}_{10}}{{\text{O}}_{4}}$ is erythritol with four alcohol groups.
Additional Information: Glycerol is known by other names also: 1,2,3-propanetriol, Glycerin, Glycerine, Glycerol Monostearate, Glyceryl Alcohol, Vegetable Glycerin.
Uses of Glycerol are-
(1) It helps the body to replace water lost during diarrhoea and vomiting, Athletes too use glycerol to keep themselves hydrated.
(2) In moisturizer creams like products, glycerol helps to bring moisture to the skin, which keeps the skin smooth. It is also used in hair conditioners, eye drops and shaving creams for the same purpose.
(3) It is added to cough syrups to help prevent irritation in the throat which causes cough.
Note: The terms molecular formula and empirical formula are almost the same but there is a little difference in these terms.
Molecular formula: Actual count of atoms present in a molecule of each element. It is the ‘true’ formula of a compound.
Example: Ethane -${{\text{C}}_{2}}{{\text{H}}_{6}}$
Empirical formula: It is the smallest/simplest ratio of atoms present in a compound.
Example: ${{(\text{C}{{\text{H}}_{3}})}_{\text{n}}}$ is the empirical formula of the alkanes. If n is equal to 2, then, the formula is ${{\text{C}}_{2}}{{\text{H}}_{6}}$ which is Ethane.
Complete step by step solution:
Glycerol is a trihydric alcohol, it contains 3 $\text{C}$ (carbon), 8$\text{H}$(hydrogen), 3$\text{O}$(oxygen) atoms in it, as suggested by the structure of glycerol. It has three alcohol groups present in it.

The molecular formula can be directly written as the count of atoms is always written in subscript after the symbol of that element.
Carbon is denoted by the symbol ‘$\text{C}$’.
Hydrogen is denoted by the symbol ‘$\text{H}$’.
Oxygen is denoted by the symbol ‘$\text{O}$’.
The molecular formula of glycerol becomes as we have both the elements in the structure, with their symbols and the count of elements present in the compound.
Molecular Formula:${{\text{C}}_{3}}{{\text{H}}_{8}}{{\text{O}}_{3}}$
The molecular formula of glycerol becomes${{\text{C}}_{3}}{{\text{H}}_{8}}{{\text{O}}_{3}}$, which is option ‘d’ which matches the answer completely.
A. $\text{C}{{\text{H}}_{3}}\text{OH}$ is methyl alcohol or methanol with 1 alcohol group present.
B. ${{\text{C}}_{2}}{{\text{H}}_{6}}{{\text{O}}_{2}}$ is ethylene glycol with two alcohol groups present.
C. ${{\text{C}}_{4}}{{\text{H}}_{10}}{{\text{O}}_{4}}$ is erythritol with four alcohol groups.
Additional Information: Glycerol is known by other names also: 1,2,3-propanetriol, Glycerin, Glycerine, Glycerol Monostearate, Glyceryl Alcohol, Vegetable Glycerin.
Uses of Glycerol are-
(1) It helps the body to replace water lost during diarrhoea and vomiting, Athletes too use glycerol to keep themselves hydrated.
(2) In moisturizer creams like products, glycerol helps to bring moisture to the skin, which keeps the skin smooth. It is also used in hair conditioners, eye drops and shaving creams for the same purpose.
(3) It is added to cough syrups to help prevent irritation in the throat which causes cough.
Note: The terms molecular formula and empirical formula are almost the same but there is a little difference in these terms.
Molecular formula: Actual count of atoms present in a molecule of each element. It is the ‘true’ formula of a compound.
Example: Ethane -${{\text{C}}_{2}}{{\text{H}}_{6}}$
Empirical formula: It is the smallest/simplest ratio of atoms present in a compound.
Example: ${{(\text{C}{{\text{H}}_{3}})}_{\text{n}}}$ is the empirical formula of the alkanes. If n is equal to 2, then, the formula is ${{\text{C}}_{2}}{{\text{H}}_{6}}$ which is Ethane.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




