
The molecular electronic configuration of \[B{e_2}\]is:
A. \[\sigma 1{s^2},\sigma *1{s^2},\sigma 2{s^2},\sigma *2{p^2}\]
B. \[\sigma 1{s^2},\sigma 2{s^2}\]
C. \[\sigma 1{s^2},\sigma *1{s^2},\sigma 2{s^2},\sigma *2{s^2}\]
D. None of the above
Answer
570k+ views
Hint: We have to remember that the electron configuration is the distribution of electrons of an atom or molecule in atomic or molecular orbitals. In order to proceed with the molecular electronic configuration, we first need to know the number of electrons in \[B{e_2}\]. Atomic number of $Be$ is $4$, hence the total number of electrons in \[B{e_2}\] is $8$. We also need to understand molecular orbitals and molecular orbital theory to be able to write the molecular electronic configuration of any molecule.
Complete step by step answer:
To begin understanding molecular orbitals, we have to first consider atomic orbitals. Let us consider the example of the simplest atom-hydrogen. We have to remember that the chemical bond is formed by the combination of two atomic orbitals.
The simplest way to obtain a molecular electronic configuration is to draw a molecular orbital diagram. Molecular orbital diagram is a plot of atomic orbitals and molecular orbitals with respect to energy. Energy is plotted vertically. The orbital with higher energy is less stable while lower energy is more stable.
In the similar way, the molecular electronic configuration of \[B{e_2}\] can be determined using molecular orbital diagram as follows:
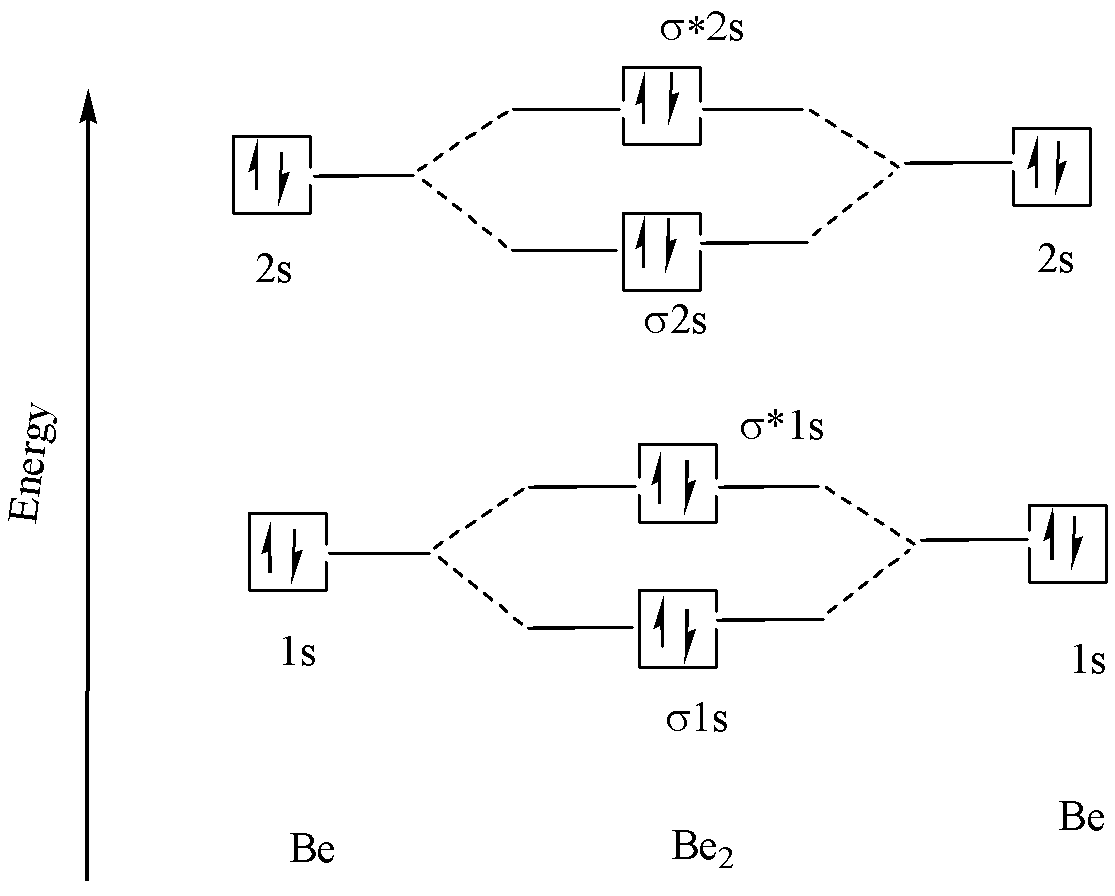
No. Of electrons in Be atom = \[4\]
Electronic configuration of Be atom = \[1{s^2}2{s^2}\]
No. Of electrons in \[B{e_2}\] molecule = $8$
Molecular electronic configuration of \[B{e_2}\] molecule = \[\sigma 1{s^2},\sigma *1{s^2},\sigma 2{s^2},\sigma *2{s^2}\].
We can calculate the bond order of beryllium using the formula as,
Bond order $ = \dfrac{1}{2}\left[ {{N_b} - {N_a}} \right]$
$ \Rightarrow {\text{Bond order}} = \dfrac{1}{2}\left[ {2 - 2} \right] = 0$
So, the correct answer is Option C.
Note: It must be noted that just as atomic orbitals are distinguished as s, p, d, and f orbitals and determined by quantum numbers. Molecular orbitals are also determined by quantum numbers and we have σ,π and δ orbitals. Molecular orbitals obey the following rules just as atomic orbitals. (a) Pauli’s Exclusion Principle: No two electrons in an atom have the same four quantum numbers. (b) Hund’s rule of maximum multiplicity: Orbitals having the same energy level should have one electron each before pairing takes place.
Complete step by step answer:
To begin understanding molecular orbitals, we have to first consider atomic orbitals. Let us consider the example of the simplest atom-hydrogen. We have to remember that the chemical bond is formed by the combination of two atomic orbitals.
The simplest way to obtain a molecular electronic configuration is to draw a molecular orbital diagram. Molecular orbital diagram is a plot of atomic orbitals and molecular orbitals with respect to energy. Energy is plotted vertically. The orbital with higher energy is less stable while lower energy is more stable.
In the similar way, the molecular electronic configuration of \[B{e_2}\] can be determined using molecular orbital diagram as follows:

No. Of electrons in Be atom = \[4\]
Electronic configuration of Be atom = \[1{s^2}2{s^2}\]
No. Of electrons in \[B{e_2}\] molecule = $8$
Molecular electronic configuration of \[B{e_2}\] molecule = \[\sigma 1{s^2},\sigma *1{s^2},\sigma 2{s^2},\sigma *2{s^2}\].
We can calculate the bond order of beryllium using the formula as,
Bond order $ = \dfrac{1}{2}\left[ {{N_b} - {N_a}} \right]$
$ \Rightarrow {\text{Bond order}} = \dfrac{1}{2}\left[ {2 - 2} \right] = 0$
So, the correct answer is Option C.
Note: It must be noted that just as atomic orbitals are distinguished as s, p, d, and f orbitals and determined by quantum numbers. Molecular orbitals are also determined by quantum numbers and we have σ,π and δ orbitals. Molecular orbitals obey the following rules just as atomic orbitals. (a) Pauli’s Exclusion Principle: No two electrons in an atom have the same four quantum numbers. (b) Hund’s rule of maximum multiplicity: Orbitals having the same energy level should have one electron each before pairing takes place.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




