
The method to prepare PMA polymer from methyl acrylate can be given as:
A. \[R - C \equiv CH\xrightarrow{{CO + ROH}}\]
B. \[HC \equiv CH\xrightarrow[{Ni{{\left( {CO} \right)}_4}}]{{CO + C{H_3}OH}}\]
C. \[HC \equiv CH\xrightarrow[{Ni{{\left( {CO} \right)}_4}}]{{CO + {H_2}O}}\]
D. None of these
Answer
595.2k+ views
Hint: This question brings in light the concept of polymers. In this question we will come across the term polymers and all the related information. We will also study what PMA polymer is, and what are its characteristics. With this information, we will be easily approaching our answer. Below here they are explained properly.
Complete step by step answer:
> Polymer: We all come in contact with many polymers in our daily lives. They are any natural or synthetic substances which in themselves include a set of very large molecules, coined as macromolecules,
> These macromolecules are those molecules which are the same and repeat themselves after and after and develop a long chain. Polymers are generally very strong as they are made by accumulation of many long chains of simpler chemical units.
> Polymers are widely available in material used by living organisms. Polymers are known as compounds of high molecular masses formed by a combination of a large number of small molecules. The phenomena of formation of polymers is known as Polymerisation.
> Poly (methyl acrylate) (PMA): This polymer is not friendly with water, it is synthetic acrylate polymer.
- PMA is rigid, leathery, and can be moulded into any shape.
- Cross linking in PMA comes in light when radiation falls on it.
- It easily gets mixed with dimethyl sulfoxide (DMSO). PMA is not so friendly with water but friendly with alkali.
- It comes into account as a macro initiator to begin the process of the copolymerisation of HEMA and DMAEMA. Also comes in account when leather finishing and textiles are considered.
- PMA polymer is made by methyl acrylate, which comes from the reaction of acetylene with \[CO\] and \[MeOH\]when \[NiC{O_4}\]is present the reaction is as shown:
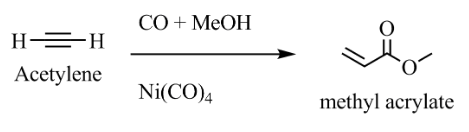
So, the correct answer is “Option B”.
Note:
1- We learned the concept of polymers which we use in our daily lives. Poly means many and mer means unit. So, from here we know what they are.
2-There are many examples of polymers present in our body like proteins, enzymes and so on. Polymers form many important components in living organisms which include cellulose, proteins, nucleic acids, etc.
Complete step by step answer:
> Polymer: We all come in contact with many polymers in our daily lives. They are any natural or synthetic substances which in themselves include a set of very large molecules, coined as macromolecules,
> These macromolecules are those molecules which are the same and repeat themselves after and after and develop a long chain. Polymers are generally very strong as they are made by accumulation of many long chains of simpler chemical units.
> Polymers are widely available in material used by living organisms. Polymers are known as compounds of high molecular masses formed by a combination of a large number of small molecules. The phenomena of formation of polymers is known as Polymerisation.
> Poly (methyl acrylate) (PMA): This polymer is not friendly with water, it is synthetic acrylate polymer.
- PMA is rigid, leathery, and can be moulded into any shape.
- Cross linking in PMA comes in light when radiation falls on it.
- It easily gets mixed with dimethyl sulfoxide (DMSO). PMA is not so friendly with water but friendly with alkali.
- It comes into account as a macro initiator to begin the process of the copolymerisation of HEMA and DMAEMA. Also comes in account when leather finishing and textiles are considered.
- PMA polymer is made by methyl acrylate, which comes from the reaction of acetylene with \[CO\] and \[MeOH\]when \[NiC{O_4}\]is present the reaction is as shown:

So, the correct answer is “Option B”.
Note:
1- We learned the concept of polymers which we use in our daily lives. Poly means many and mer means unit. So, from here we know what they are.
2-There are many examples of polymers present in our body like proteins, enzymes and so on. Polymers form many important components in living organisms which include cellulose, proteins, nucleic acids, etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




