
The maximum number of isomers (including stereoisomers) that are possible on mono-chlorination of the following compound, is:
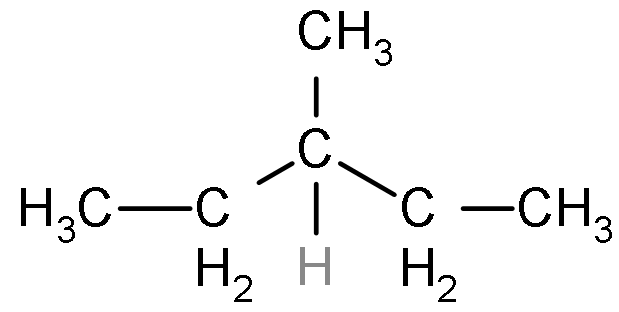
A) 8
B) 9
C) 10
D) 11

Answer
581.7k+ views
Hint: For this problem, firstly we have to write all the products or isomers which are possible when one chlorine atom replaces a hydrogen atom in the structure and after it, those who have enantiomer will have 2 isomers and those which do not have chiral carbon will form only 1 isomer.
Complete step by step solution:
- In the given question, we have to explain the total number of isomers which will be formed when the monochlorination of the compound will be done.
- As we know that the monochlorination is the process of the addition of the halide such as chlorine, bromine, iodine, etc by the removal of the hydrogen atom.
- If the monochlorinated product formed is an enantiomer then it will give us two types of molecules because the molecule formed will be non-superimposable mirror image.
- So, as we can see that there are four possibilities of the chlorine atom to attach on the structure by the removal of the hydrogen atom.
- Firstly, the chlorine atom will attach on the first carbon from the left side and another product can be formed by the attachment of chlorine on the second carbon.
- Similarly, the third product can be formed by the attachment of the chlorine on the central third carbon.
- And the last product can be formed by the attachment of the chlorine on the side chain methyl group as shown below:
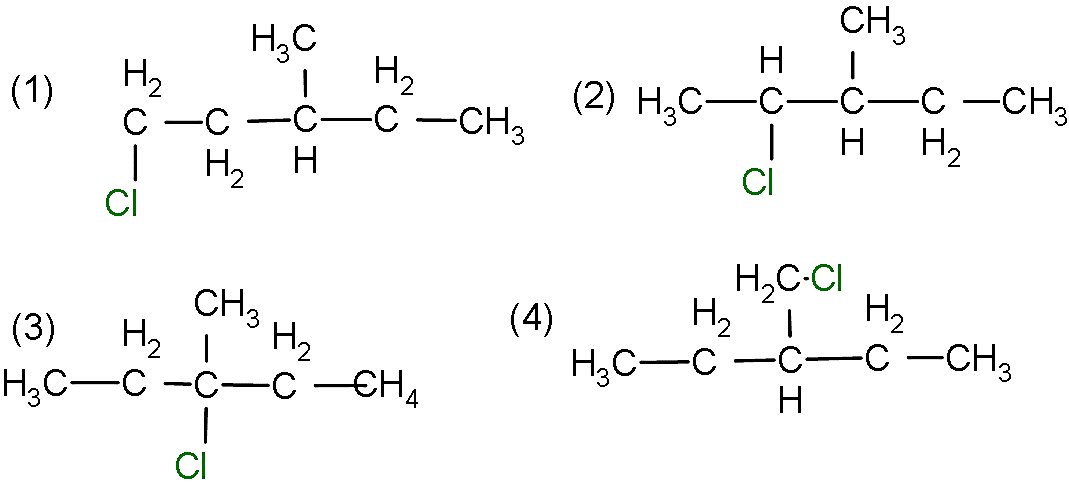
- So, here the first structure will have enantiomer and it will give two molecules whereas the second structure has two chiral centres and it will give 4 molecules.
- Whereas the structure 3 and 4 don’t give enantiomer structure so the total number of monochlorinated products will be: $2 + 4 + 1 + 1 = 8$.
Therefore, option A) is the correct answer.
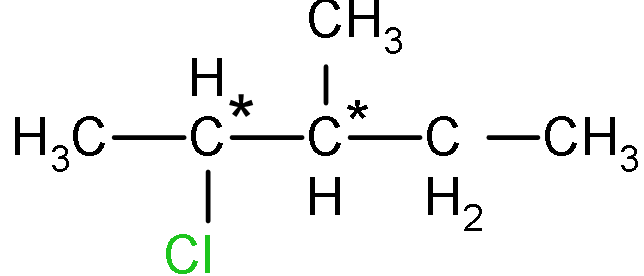
Note: In the given problem, the chiral centre is the carbon from which four different groups are attached and the second structure has two chiral centres that's why it will give four structures as shown below:
Complete step by step solution:
- In the given question, we have to explain the total number of isomers which will be formed when the monochlorination of the compound will be done.
- As we know that the monochlorination is the process of the addition of the halide such as chlorine, bromine, iodine, etc by the removal of the hydrogen atom.
- If the monochlorinated product formed is an enantiomer then it will give us two types of molecules because the molecule formed will be non-superimposable mirror image.
- So, as we can see that there are four possibilities of the chlorine atom to attach on the structure by the removal of the hydrogen atom.
- Firstly, the chlorine atom will attach on the first carbon from the left side and another product can be formed by the attachment of chlorine on the second carbon.
- Similarly, the third product can be formed by the attachment of the chlorine on the central third carbon.
- And the last product can be formed by the attachment of the chlorine on the side chain methyl group as shown below:

- So, here the first structure will have enantiomer and it will give two molecules whereas the second structure has two chiral centres and it will give 4 molecules.
- Whereas the structure 3 and 4 don’t give enantiomer structure so the total number of monochlorinated products will be: $2 + 4 + 1 + 1 = 8$.
Therefore, option A) is the correct answer.
Note: In the given problem, the chiral centre is the carbon from which four different groups are attached and the second structure has two chiral centres that's why it will give four structures as shown below:

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




