
The maximum covalency of oxygen in ozone is :
A.$5$
B.$3$
C.$2$
D.$4$
Answer
584.7k+ views
Hint: In any compound , elements share their electrons with other atoms of the same or different element to obtain stable electronic configuration . The number of electrons shared by the elements to get stable configuration is known as covalency.
Complete step by step answer:
Ozone is a form of elemental oxygen. It is a very unstable form of oxygen. Most stable form of oxygen is its diatomic molecule i.e. ${O_2}$ . It is found naturally in the Earth’s upper atmosphere.
Ozone is also known as tri oxygen which is an inorganic molecule and its colour is pale blue and possesses a pungent smell. It is a lot of oxygen.
Structural formula of compound is given the figure: Central oxygen atom i.e. ${O^ + }$ shares its three electrons and other two atoms share two and one electron respectively . Hence maximum covalency of oxygen in ozone is three.
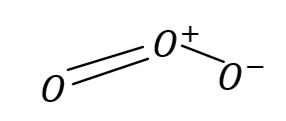
So, the correct answer is Option B .
Note:
Ozone saves us from the harmful UV rays of the sun . Ozone also shows resonance and it has two resonating structures, they contribute equally to the hybrid structure . Boiling point of ozone is $ - 112^\circ C$ and it is soluble in water , sulphuric acid and carbon tetrachloride . Ozone is harmful for humans as it can damage the lungs, it can cause chest pain , coughing and throat irritation . It is found in the stratosphere which is the second layer of the Earth’s atmosphere as it protects human beings from harmful UV rays.
Complete step by step answer:
Ozone is a form of elemental oxygen. It is a very unstable form of oxygen. Most stable form of oxygen is its diatomic molecule i.e. ${O_2}$ . It is found naturally in the Earth’s upper atmosphere.
Ozone is also known as tri oxygen which is an inorganic molecule and its colour is pale blue and possesses a pungent smell. It is a lot of oxygen.
Structural formula of compound is given the figure: Central oxygen atom i.e. ${O^ + }$ shares its three electrons and other two atoms share two and one electron respectively . Hence maximum covalency of oxygen in ozone is three.

So, the correct answer is Option B .
Note:
Ozone saves us from the harmful UV rays of the sun . Ozone also shows resonance and it has two resonating structures, they contribute equally to the hybrid structure . Boiling point of ozone is $ - 112^\circ C$ and it is soluble in water , sulphuric acid and carbon tetrachloride . Ozone is harmful for humans as it can damage the lungs, it can cause chest pain , coughing and throat irritation . It is found in the stratosphere which is the second layer of the Earth’s atmosphere as it protects human beings from harmful UV rays.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




