
The major product ‘Y’ in the following reaction is:
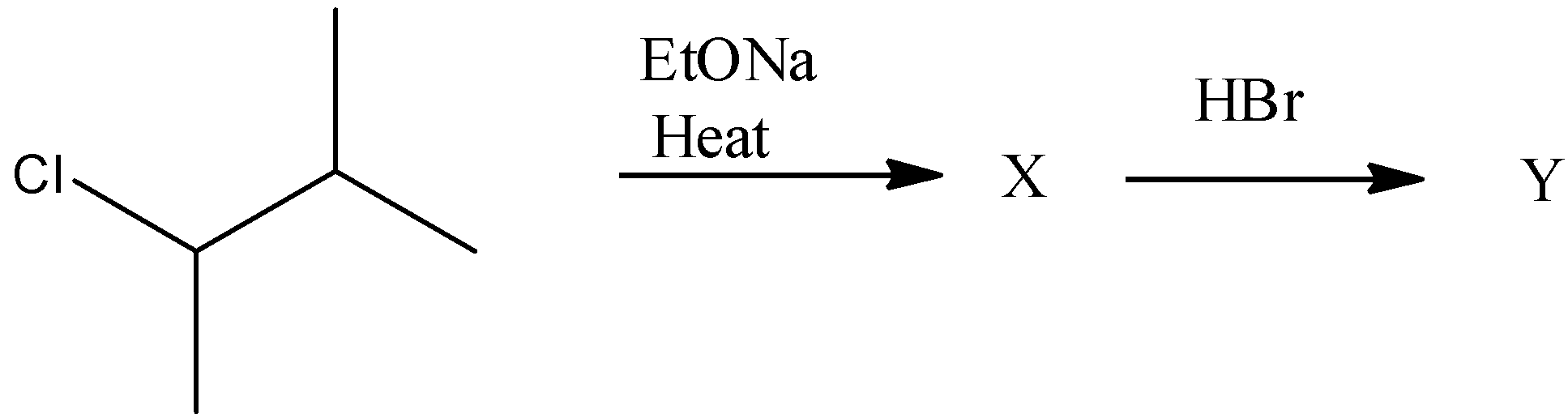
(A)
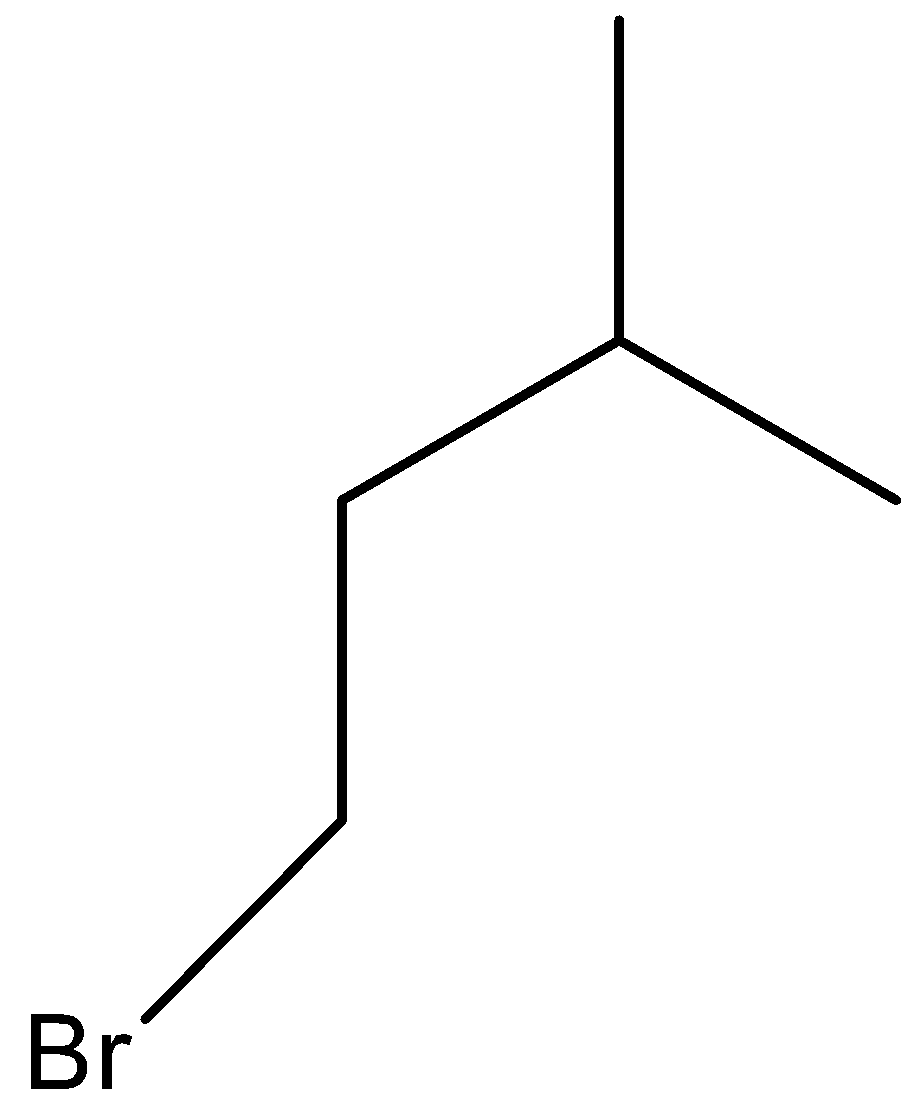
(B)
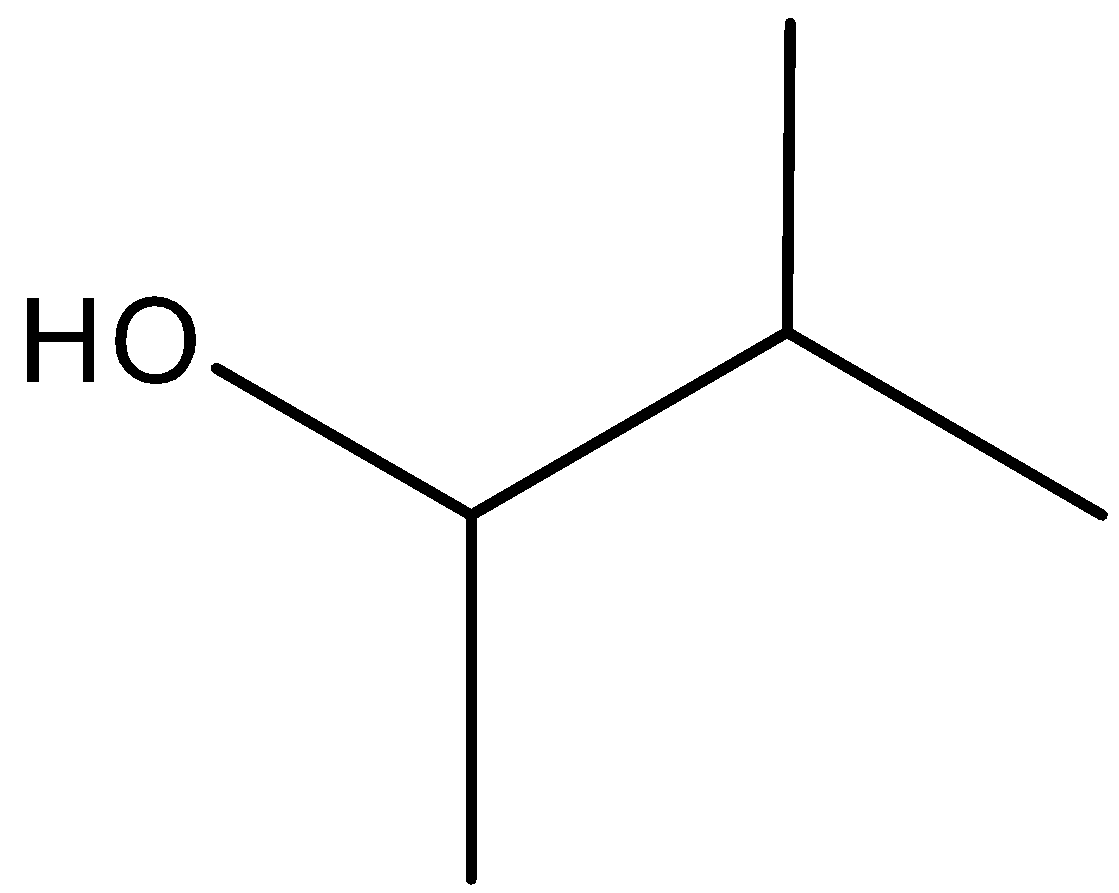
(C)
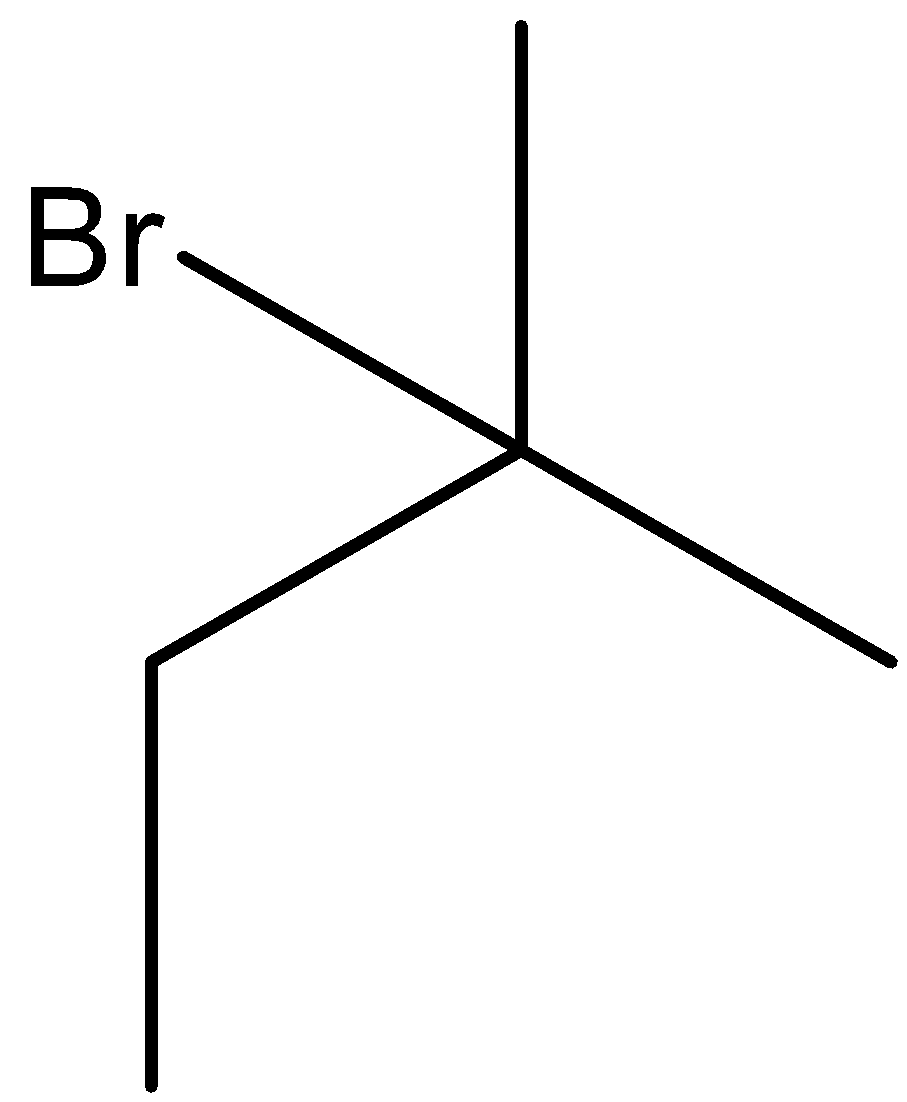
(D)





Answer
578.1k+ views
Hint:. In the first step of the reaction, in presence of sodium ethoxide HCl will be removed. So, dehydrohalogenation reactions will take place. In the second place, there will be an addition reaction.
Complete step by step answer:
Let’s see the complete reaction in order to find the answer.
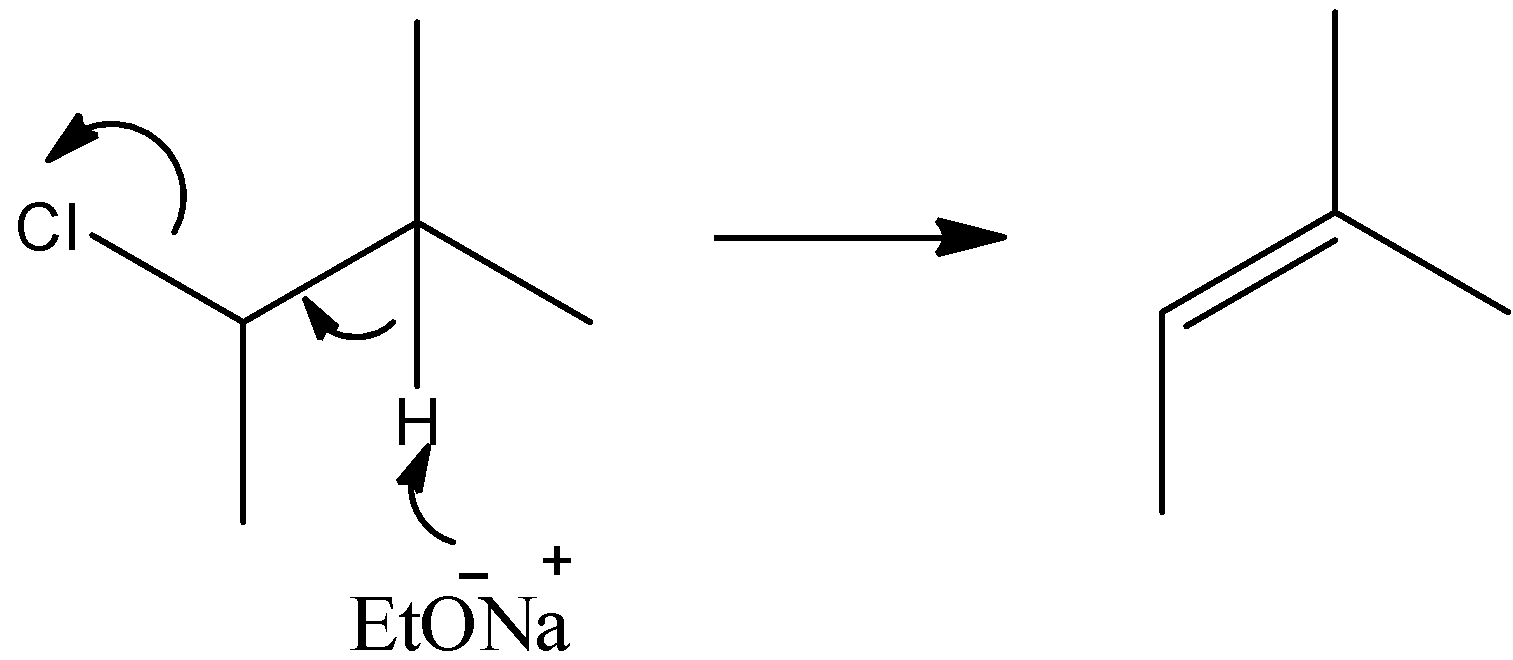
- We have an alkyl halide as a starting material. It is a secondary chloride. It reacts with Sodium ethoxide (NaOEt) which is a strong base. So, a strong base will remove the halogen atom and a hydrogen atom from the $\alpha $-carbon. Thus, we can say that dehydrohalogenation reactions will occur here. Here, the hydrogen atom of tertiary carbon will get removed. The mechanism of the reaction can be given by
Then, the resultant alkene is allowed to react with HBr. We know that hydrogen atom is positively charged and bromine atom is negatively charged in HBr.
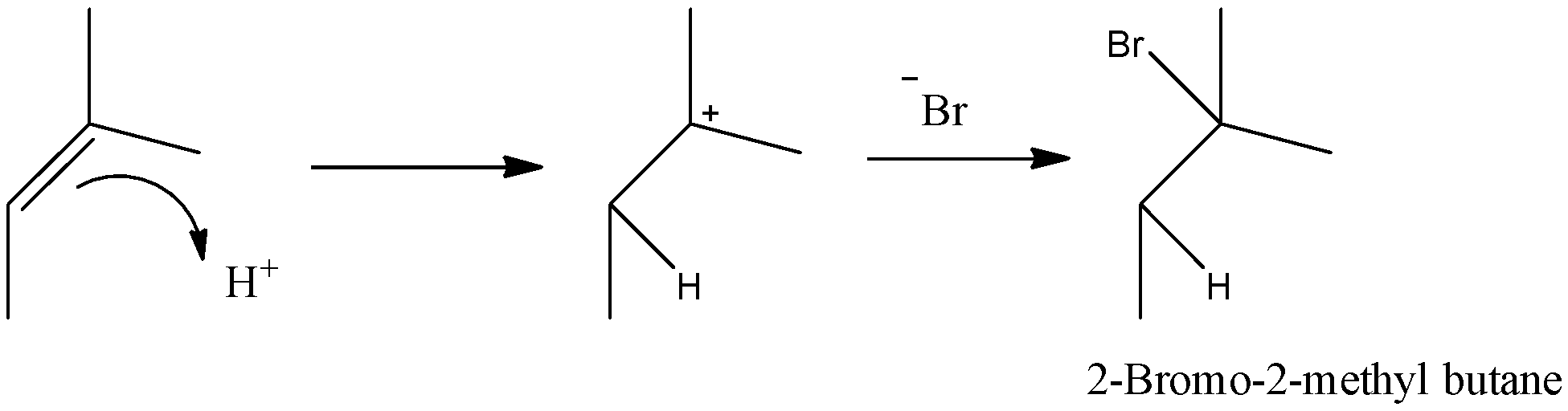
- So, hydrogen atoms will be attacked in a way that more stable carbocation is formed. We can see that there is a possibility of formation of a secondary or tertiary carbocation. So, as tertiary carbocation is more stable, it will be formed. This will react with negatively charged bromine ion to give 2-bromo-2-methyl butane as a product. The mechanism can be given as
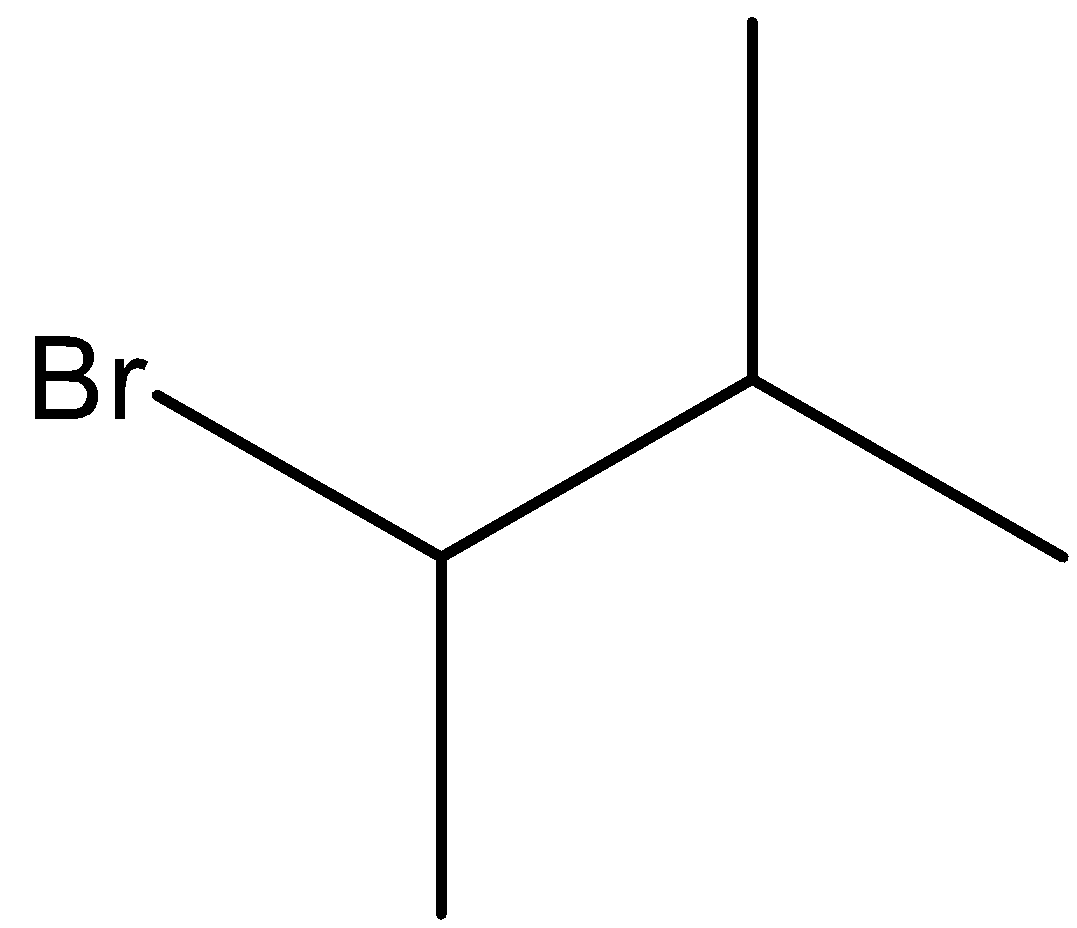
Thus, we can say that Y in the given reaction is 2-bromo-2-methyl butane.
So, the correct answer is “Option C”.
Note: Remember that in dehydrohalogenation reaction, the elimination of hydrogen takes place in a way that more substituted alkene gets formed. In addition reaction, the more electronegative atom gets attached to the carbon having the least number of hydrogen atoms which is also called Markovnikov’s rule.
Complete step by step answer:
Let’s see the complete reaction in order to find the answer.
- We have an alkyl halide as a starting material. It is a secondary chloride. It reacts with Sodium ethoxide (NaOEt) which is a strong base. So, a strong base will remove the halogen atom and a hydrogen atom from the $\alpha $-carbon. Thus, we can say that dehydrohalogenation reactions will occur here. Here, the hydrogen atom of tertiary carbon will get removed. The mechanism of the reaction can be given by

Then, the resultant alkene is allowed to react with HBr. We know that hydrogen atom is positively charged and bromine atom is negatively charged in HBr.
- So, hydrogen atoms will be attacked in a way that more stable carbocation is formed. We can see that there is a possibility of formation of a secondary or tertiary carbocation. So, as tertiary carbocation is more stable, it will be formed. This will react with negatively charged bromine ion to give 2-bromo-2-methyl butane as a product. The mechanism can be given as

Thus, we can say that Y in the given reaction is 2-bromo-2-methyl butane.
So, the correct answer is “Option C”.
Note: Remember that in dehydrohalogenation reaction, the elimination of hydrogen takes place in a way that more substituted alkene gets formed. In addition reaction, the more electronegative atom gets attached to the carbon having the least number of hydrogen atoms which is also called Markovnikov’s rule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




