
The major product (s) of the following reaction is (are):
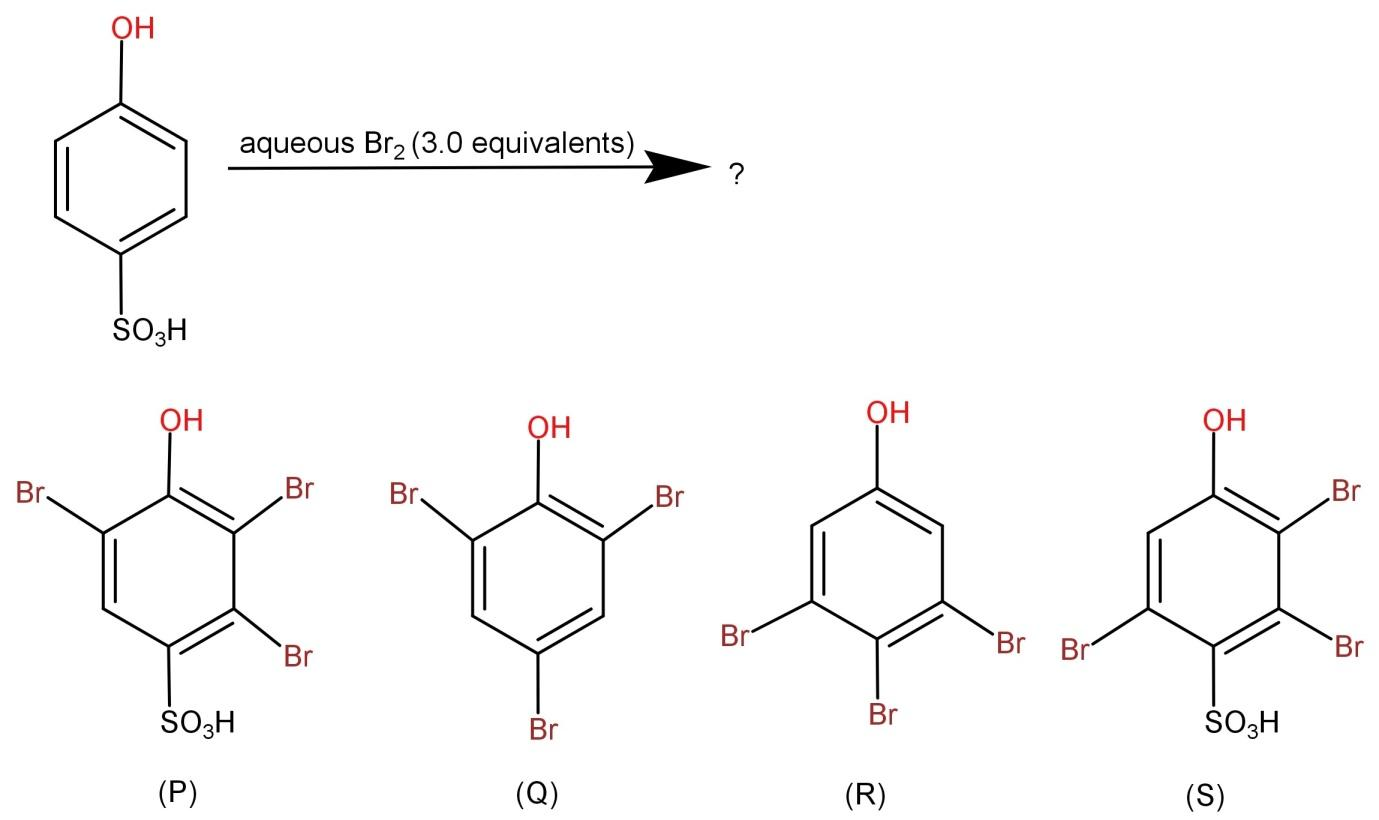

Answer
579.6k+ views
Hint: The hydroxyl group (-OH) is ortho and para directing while the sulfonyl group ($ - S{O_3}H$) is a group deactivating group and is a good leaving group.
Complete step by step answer:
-First of all let us see the reactant and the nature of groups attached to it.
The given reactant in the question has 2 groups: a hydroxyl group (-OH) and sulfonyl group ($ - S{O_3}H$). We all should know that the hydroxyl group (-OH) is a ring activating group and is also ortho and para directing. But the sulfonyl group ($ - S{O_3}H$) is a ring deactivating group and it is a good leaving group.
-When the reactant reacts with aqueous bromine, there occurs electrophilic substitution reaction and the hydroxyl group (phenoxide ion) in the aqueous solution becomes extremely reactive.
-First the bromine reacts with the ring and bromine atoms get attached to both the ortho and para position. The para position is shared by a bromine atom and the sulfonyl group and the carbon at the meta position has a positive charge (electrophilic carbon). Next the sulfonyl group being a good leaving group leaves the para position where the bond gets transferred to the ring. Hence the final product formed is: 2,4,6-tribromophenol and it is a white ppt.
In short we can say that the sulfonyl group ($ - S{O_3}H$) being a good leaving group is easily replaced by bromine atoms.
-The reaction occurs in the following manner:
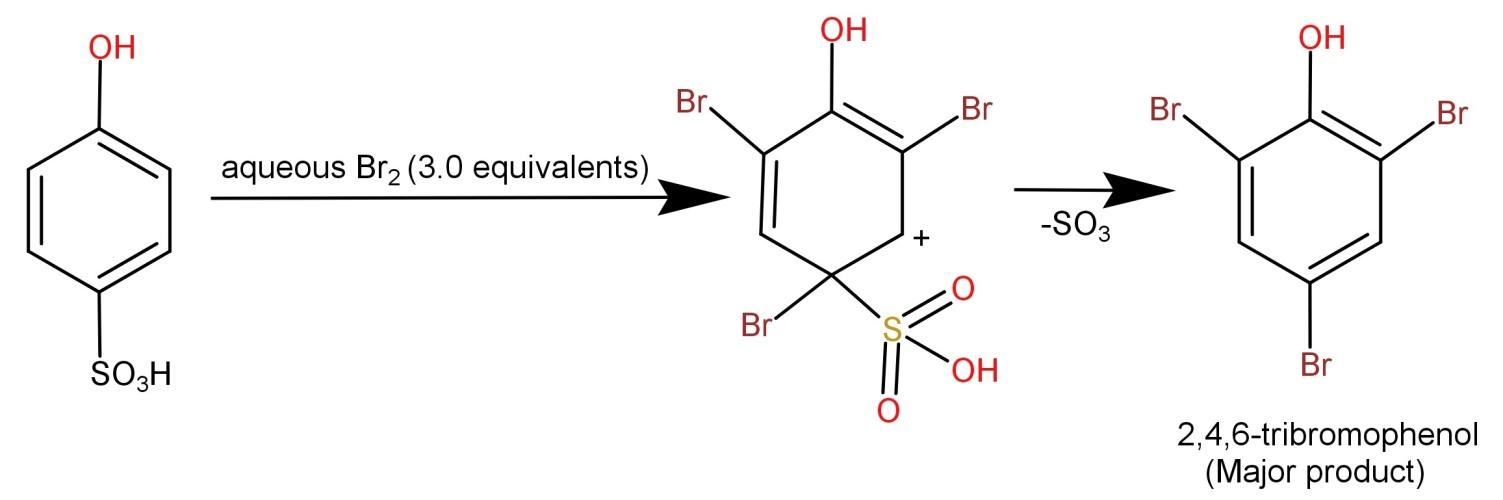
-Hence, we can say that the major product is 2,4,6-tribromophenol.
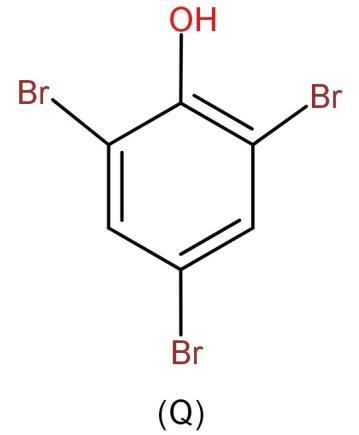
So, the correct answer is “Option Q”.
Note: A leaving group is basically a group or a molecule which leaves easily along with a pair of electrons from a heterolytic bond cleavage. They can be anions, cations or even neutral molecules. Basically the weak bases are the best leaving groups. For example: halide ions like $C{l^ - }$, $B{r^ - }$, ${I^ - }$, etc. The weaker the base, the better is the leaving group.
Complete step by step answer:
-First of all let us see the reactant and the nature of groups attached to it.
The given reactant in the question has 2 groups: a hydroxyl group (-OH) and sulfonyl group ($ - S{O_3}H$). We all should know that the hydroxyl group (-OH) is a ring activating group and is also ortho and para directing. But the sulfonyl group ($ - S{O_3}H$) is a ring deactivating group and it is a good leaving group.
-When the reactant reacts with aqueous bromine, there occurs electrophilic substitution reaction and the hydroxyl group (phenoxide ion) in the aqueous solution becomes extremely reactive.
-First the bromine reacts with the ring and bromine atoms get attached to both the ortho and para position. The para position is shared by a bromine atom and the sulfonyl group and the carbon at the meta position has a positive charge (electrophilic carbon). Next the sulfonyl group being a good leaving group leaves the para position where the bond gets transferred to the ring. Hence the final product formed is: 2,4,6-tribromophenol and it is a white ppt.
In short we can say that the sulfonyl group ($ - S{O_3}H$) being a good leaving group is easily replaced by bromine atoms.
-The reaction occurs in the following manner:

-Hence, we can say that the major product is 2,4,6-tribromophenol.

So, the correct answer is “Option Q”.
Note: A leaving group is basically a group or a molecule which leaves easily along with a pair of electrons from a heterolytic bond cleavage. They can be anions, cations or even neutral molecules. Basically the weak bases are the best leaving groups. For example: halide ions like $C{l^ - }$, $B{r^ - }$, ${I^ - }$, etc. The weaker the base, the better is the leaving group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




