
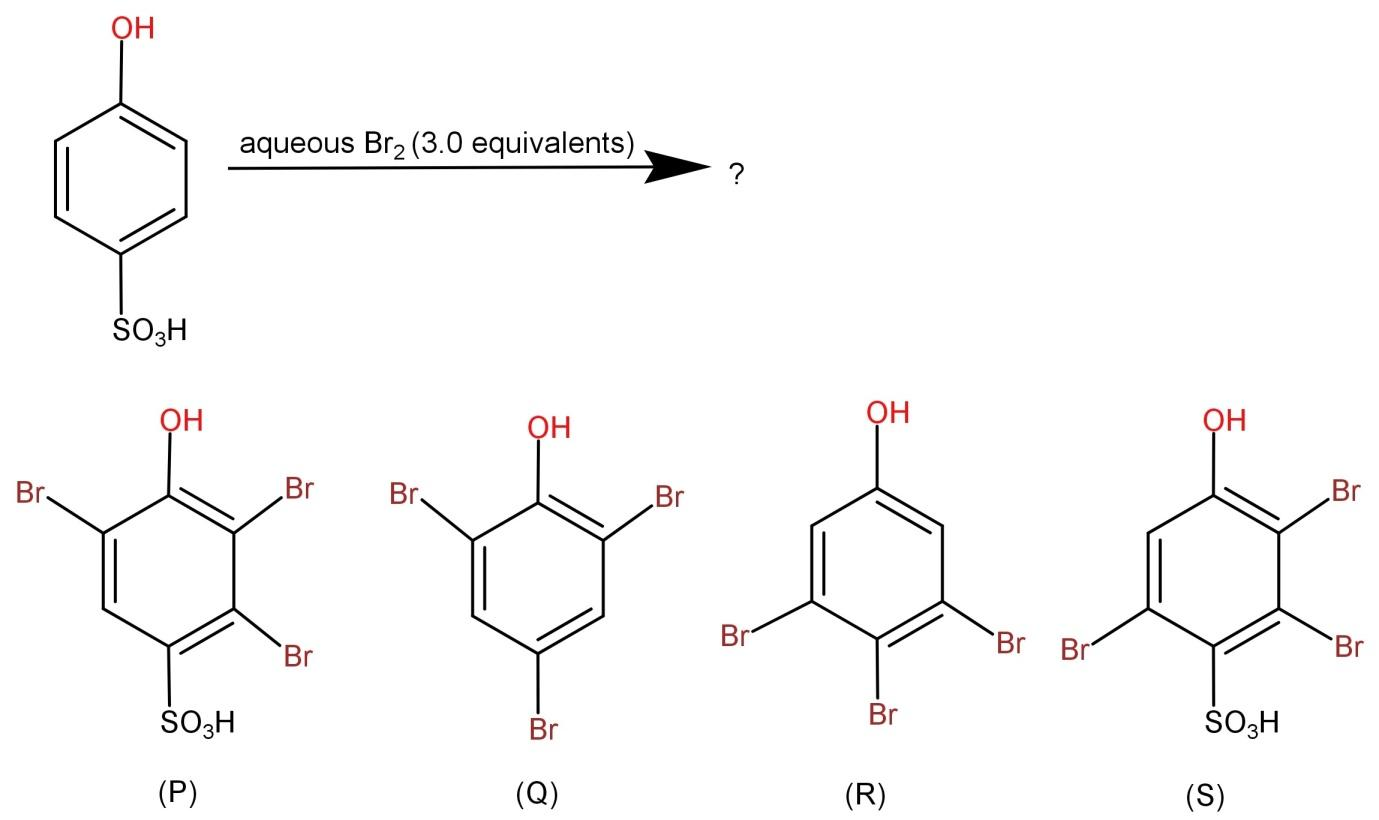
The major product (s) of the following reaction is (are):

Answer
588.6k+ views
Hint: The hydroxyl group (-OH) is ortho and para directing while the sulfonyl group ($ - S{O_3}H$) is a group deactivating group and is a good leaving group.
Complete step by step answer:
-First of all let us see the reactant and the nature of groups attached to it.
The given reactant in the question has 2 groups: a hydroxyl group (-OH) and sulfonyl group ($ - S{O_3}H$). We all should know that the hydroxyl group (-OH) is a ring activating group and is also ortho and para directing. But the sulfonyl group ($ - S{O_3}H$) is a ring deactivating group and it is a good leaving group.
-When the reactant reacts with aqueous bromine, there occurs electrophilic substitution reaction and the hydroxyl group (phenoxide ion) in the aqueous solution becomes extremely reactive.
-First the bromine reacts with the ring and bromine atoms get attached to both the ortho and para position. The para position is shared by a bromine atom and the sulfonyl group and the carbon at the meta position has a positive charge (electrophilic carbon). Next the sulfonyl group being a good leaving group leaves the para position where the bond gets transferred to the ring. Hence the final product formed is: 2,4,6-tribromophenol and it is a white ppt.
In short we can say that the sulfonyl group ($ - S{O_3}H$) being a good leaving group is easily replaced by bromine atoms.
-The reaction occurs in the following manner:
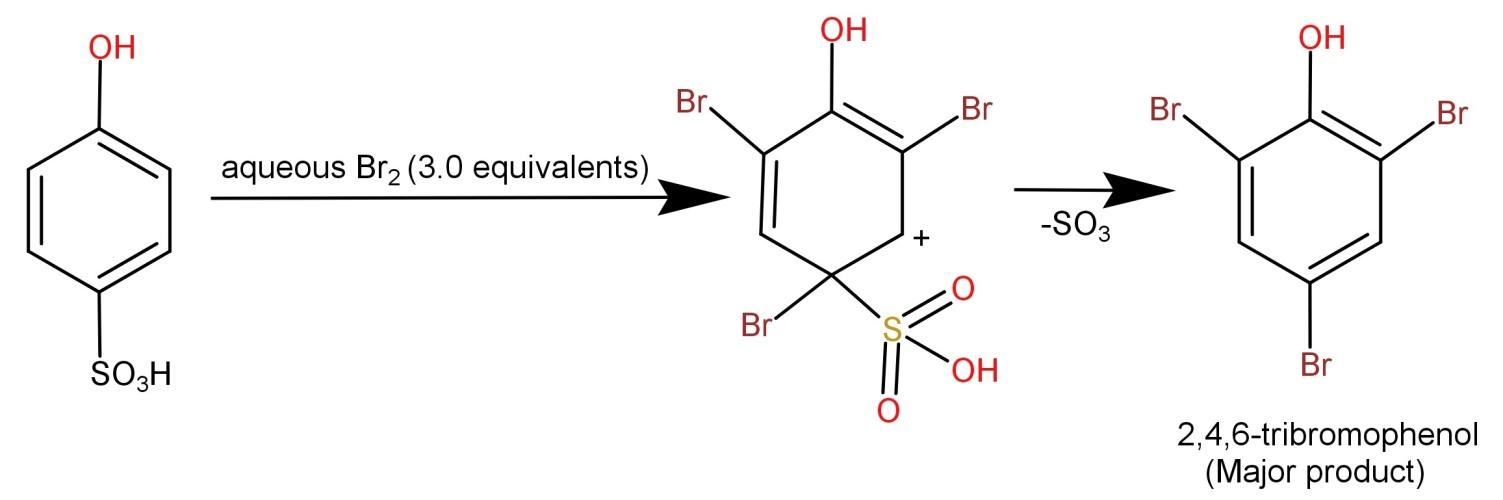
-Hence, we can say that the major product is 2,4,6-tribromophenol.
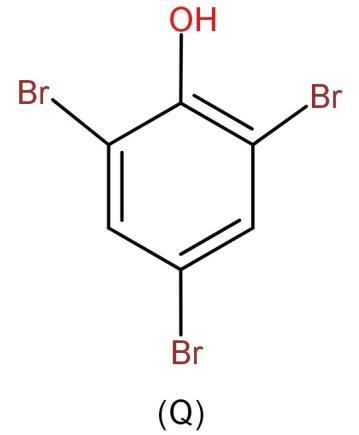
So, the correct answer is “Option Q”.
Note: A leaving group is basically a group or a molecule which leaves easily along with a pair of electrons from a heterolytic bond cleavage. They can be anions, cations or even neutral molecules. Basically the weak bases are the best leaving groups. For example: halide ions like $C{l^ - }$, $B{r^ - }$, ${I^ - }$, etc. The weaker the base, the better is the leaving group.
Complete step by step answer:
-First of all let us see the reactant and the nature of groups attached to it.
The given reactant in the question has 2 groups: a hydroxyl group (-OH) and sulfonyl group ($ - S{O_3}H$). We all should know that the hydroxyl group (-OH) is a ring activating group and is also ortho and para directing. But the sulfonyl group ($ - S{O_3}H$) is a ring deactivating group and it is a good leaving group.
-When the reactant reacts with aqueous bromine, there occurs electrophilic substitution reaction and the hydroxyl group (phenoxide ion) in the aqueous solution becomes extremely reactive.
-First the bromine reacts with the ring and bromine atoms get attached to both the ortho and para position. The para position is shared by a bromine atom and the sulfonyl group and the carbon at the meta position has a positive charge (electrophilic carbon). Next the sulfonyl group being a good leaving group leaves the para position where the bond gets transferred to the ring. Hence the final product formed is: 2,4,6-tribromophenol and it is a white ppt.
In short we can say that the sulfonyl group ($ - S{O_3}H$) being a good leaving group is easily replaced by bromine atoms.
-The reaction occurs in the following manner:

-Hence, we can say that the major product is 2,4,6-tribromophenol.

So, the correct answer is “Option Q”.
Note: A leaving group is basically a group or a molecule which leaves easily along with a pair of electrons from a heterolytic bond cleavage. They can be anions, cations or even neutral molecules. Basically the weak bases are the best leaving groups. For example: halide ions like $C{l^ - }$, $B{r^ - }$, ${I^ - }$, etc. The weaker the base, the better is the leaving group.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Give 10 examples of unisexual and bisexual flowers

Give simple chemical tests to distinguish between the class 12 chemistry CBSE

Define Vant Hoff factor How is it related to the degree class 12 chemistry CBSE




