
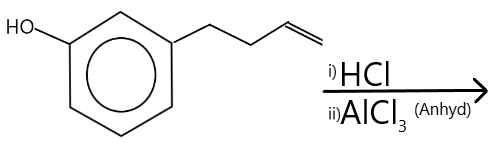
The major product of the following reaction is:

Answer
558.9k+ views
Hint:We will first identify the functional group in the reactant and the group that will react with the given reagents. The functional group will not be altered because of the reagents due to the resonance effect.
Complete solution:
We can see that the $OH$ group is the functional group in the reactant, and is directly bonded with the benzene group, this direct bonding will cause resonance and the $C - O$ will become stronger, and hence there will be no direct reaction of the given reagents and the functional group ($OH$).
However, the butene (or butylene) group bonded at the meta position will react with the $HCl$, and anhydrous ${\text{AlC}}{{\text{l}}_{\text{3}}}$ to yield a product following Markovnikov’s rule. The reaction and be written as:

Additional information:
According to Markovnikov’s rule, the negative part of an $H - X(X = Cl,Br,I)$ will get bonded to the carbon having less number of hydrogen.
However, the above condition can be altered and the reaction can be altered by adding peroxide. When peroxide is added to the reaction mixture the negative part will make a bond with the carbon having more hydrogen.
Note: It is possible to form aryl halides from aliphatic alcohol, but with phenols, it is not possible because of the resonance effect, and due to this effect the $C - O$ bond becomes $C = O$ in some resonating structures, this double bond is stronger than the single bond and hence cleavage of $C - O$ is not possible in phenols.
Complete solution:
We can see that the $OH$ group is the functional group in the reactant, and is directly bonded with the benzene group, this direct bonding will cause resonance and the $C - O$ will become stronger, and hence there will be no direct reaction of the given reagents and the functional group ($OH$).
However, the butene (or butylene) group bonded at the meta position will react with the $HCl$, and anhydrous ${\text{AlC}}{{\text{l}}_{\text{3}}}$ to yield a product following Markovnikov’s rule. The reaction and be written as:

Additional information:
According to Markovnikov’s rule, the negative part of an $H - X(X = Cl,Br,I)$ will get bonded to the carbon having less number of hydrogen.
However, the above condition can be altered and the reaction can be altered by adding peroxide. When peroxide is added to the reaction mixture the negative part will make a bond with the carbon having more hydrogen.
Note: It is possible to form aryl halides from aliphatic alcohol, but with phenols, it is not possible because of the resonance effect, and due to this effect the $C - O$ bond becomes $C = O$ in some resonating structures, this double bond is stronger than the single bond and hence cleavage of $C - O$ is not possible in phenols.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




