
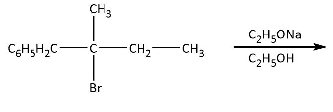
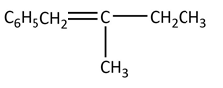
The major product of the following reaction is:
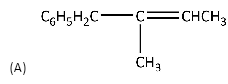
(B)
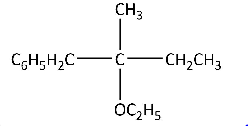
(C)
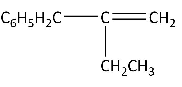
(D)





Answer
564.3k+ views
Hint:As we know that sodium ethanoate is a base as well as a nucleophile. which is not bulky because the bulky base always attacks at the site where the electron density is very less so, that they give the most stable product.
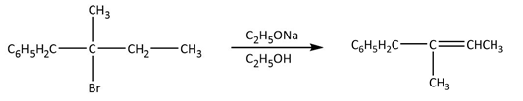
Complete step-by-step answer:In this reaction, sodium ethanoate is acting as a base not a nucleophile, let’s clear this statement from the options-
Sodium ethanoate is not a bulky base because it is not substituted with bulky groups so it can
abstract most acidic proton which can eliminate hydrogen bromide and can form alkene.
But according to Saytzeff’s rule, with base, substituted alkene will be the most stable alkene, so
sodium ethanoate abstracts methyl hydrogen which is placed at third carbon and gives the most
stable alkene. The complete reaction is shown below.
Option (B) will not be the product because sodium ethanoate will feel repulsion due to more
electron density around the bromine atom.
Option (C) will not give stable alkene because the product will not be substituted alkene.
Option (D) will not give stable alkene as the anion which is formed after abstraction is not stable.
Therefore, the option (A) is the correct option because it gives substituted alkene.
Note:Hoffmann rule gives the least substituted alkene because base which is used in this reaction is very bulky in nature which abstracts the acidic proton which has the least electron density.
Complete step-by-step answer:In this reaction, sodium ethanoate is acting as a base not a nucleophile, let’s clear this statement from the options-
Sodium ethanoate is not a bulky base because it is not substituted with bulky groups so it can
abstract most acidic proton which can eliminate hydrogen bromide and can form alkene.
But according to Saytzeff’s rule, with base, substituted alkene will be the most stable alkene, so
sodium ethanoate abstracts methyl hydrogen which is placed at third carbon and gives the most
stable alkene. The complete reaction is shown below.

Option (B) will not be the product because sodium ethanoate will feel repulsion due to more
electron density around the bromine atom.
Option (C) will not give stable alkene because the product will not be substituted alkene.
Option (D) will not give stable alkene as the anion which is formed after abstraction is not stable.
Therefore, the option (A) is the correct option because it gives substituted alkene.
Note:Hoffmann rule gives the least substituted alkene because base which is used in this reaction is very bulky in nature which abstracts the acidic proton which has the least electron density.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




