
The major product of the following reaction is:
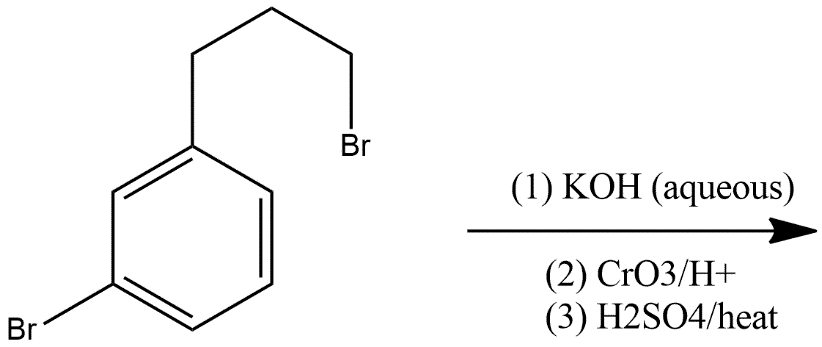
A.

B.
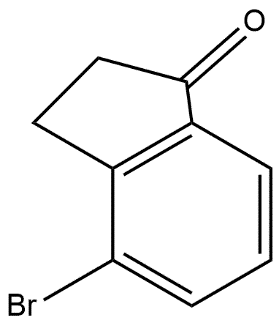
C.
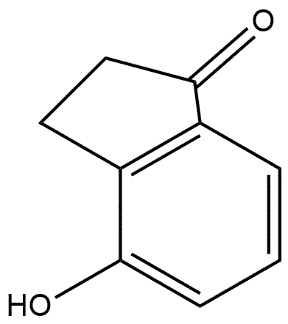
D.





Answer
578.4k+ views
Hint: The reagent present in the reaction is generally a zone’s reagent which is prepared by dissolving chromium trioxide in aqueous sulfuric acid this type of oxidation reactions are very rapid and exothermic in nature.
Complete step by step answer:
This is generally an oxidation reaction in which oxidation process. The reagent present is a zone’s reagent. By the use of this reagent, primary alcohol is converted into acids, secondary alcohol to ketones and in this case 1,2-diols suffer from fragmentation. Allylic alcohols are readily oxidized while alkenes and alkynes are resistant and it does not undergo zone’s reagent oxidation.
In the given reaction firstly KOH attacks on bromine atom and it gets converted into alcohol one which can be shown as:
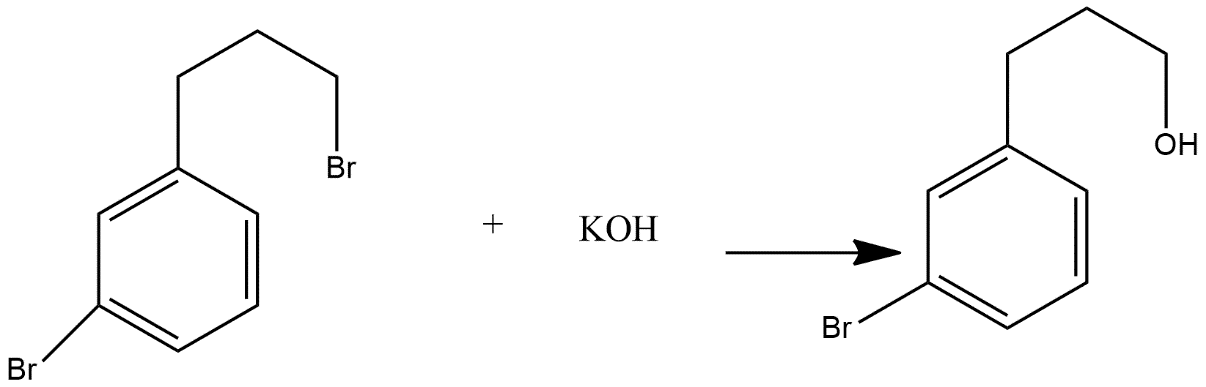
And after that ring closure process is done which converts the alcoholic product in to ketone and the product formed is as given in option B i.e.
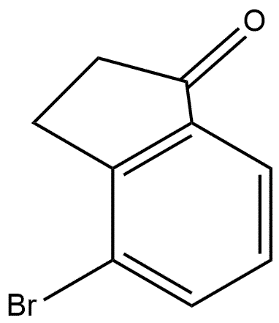
So, the correct answer is “Option B”.
Note: The reagent can also be prepared from sodium dichromate and potassium dichromate. Although the reagent is very acidic and the substrate in acetone is essentially titrated with the oxidant solution and only very acid-sensitive groups are incompatible. For example esters, even tert-butyl esters, remain unchanged. The concentration of sulfuric acid can be decreased to minimize side reactions, although the oxidation power decreases too.
Complete step by step answer:
This is generally an oxidation reaction in which oxidation process. The reagent present is a zone’s reagent. By the use of this reagent, primary alcohol is converted into acids, secondary alcohol to ketones and in this case 1,2-diols suffer from fragmentation. Allylic alcohols are readily oxidized while alkenes and alkynes are resistant and it does not undergo zone’s reagent oxidation.
In the given reaction firstly KOH attacks on bromine atom and it gets converted into alcohol one which can be shown as:

And after that ring closure process is done which converts the alcoholic product in to ketone and the product formed is as given in option B i.e.

So, the correct answer is “Option B”.
Note: The reagent can also be prepared from sodium dichromate and potassium dichromate. Although the reagent is very acidic and the substrate in acetone is essentially titrated with the oxidant solution and only very acid-sensitive groups are incompatible. For example esters, even tert-butyl esters, remain unchanged. The concentration of sulfuric acid can be decreased to minimize side reactions, although the oxidation power decreases too.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




