
The major product B is:

Answer
575.1k+ views
Hint:First of all, the product is formed as a nucleophilic attack on ortho position with respect to the nitro group and then acid is formed on the same place after hydrolysis in the presence of acid.
Complete step by step solution:
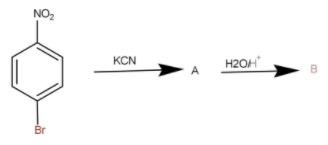
The reactant given to us is known as para-bromonitrobenzene. The reaction of para bromonitrobenzene with potassium cyanide is named as von Richter reaction. This is a nucleophilic substitution reaction in which one nucleophile substitutes another group present on the ring. The cyanide group substitute \[{\text{N}}{{\text{O}}_2}\] group as \[{\text{N}}{{\text{O}}_2}\] is a good leaving group. The product formed is at ortho position instead of para position. The mechanism followed is a complex mechanism and hence we need not to write the mechanism. Product formed is as follow:
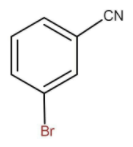
Then the hydrolysis of compounds occurs in the presence of an acid. Then the cyanide gets converted into carboxylic acid at the same position as of cyanide. The performed formed is as follow:
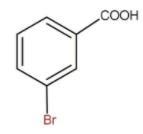
The name of the product formed is meta bromo benzoic acid.
Actually one more product is formed during the above reaction that is p bromobenzoic acid but we consider meta bromobenzoic acid as the major product and the p bromobenzoic acid as the minor product.
Note:
A substitution reaction is a reaction in which one group replaces the other group. The better the leaving group is the easier will be the substitution. Potassium cyanide is a colorless crystalline compound. It is mainly used in organic compound synthesis, mining of gold, and electroplating. Since it gives free cyanide and so is toxic in nature.
Complete step by step solution:
The reactant given to us is known as para-bromonitrobenzene. The reaction of para bromonitrobenzene with potassium cyanide is named as von Richter reaction. This is a nucleophilic substitution reaction in which one nucleophile substitutes another group present on the ring. The cyanide group substitute \[{\text{N}}{{\text{O}}_2}\] group as \[{\text{N}}{{\text{O}}_2}\] is a good leaving group. The product formed is at ortho position instead of para position. The mechanism followed is a complex mechanism and hence we need not to write the mechanism. Product formed is as follow:

Then the hydrolysis of compounds occurs in the presence of an acid. Then the cyanide gets converted into carboxylic acid at the same position as of cyanide. The performed formed is as follow:

The name of the product formed is meta bromo benzoic acid.
Actually one more product is formed during the above reaction that is p bromobenzoic acid but we consider meta bromobenzoic acid as the major product and the p bromobenzoic acid as the minor product.
Note:
A substitution reaction is a reaction in which one group replaces the other group. The better the leaving group is the easier will be the substitution. Potassium cyanide is a colorless crystalline compound. It is mainly used in organic compound synthesis, mining of gold, and electroplating. Since it gives free cyanide and so is toxic in nature.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




