
The light yellow compound produced when acetone reacts with iodine and alkali is,
(A) $ C{H_3}.CO.C{H_2}I $
(B) $ C{H_3}I $
(C) $ CH{I_3} $
(D) None of these
Answer
547.2k+ views
Hint: When acetone reacts with iodine in the presence of alkali, iodoform is formed which is a light yellow color precipitate and the reaction is known as iodoform reaction. Iodoform test is a qualitative test, meaning it can be used for the verification of specific quality or characteristics. Basically, it is used for the detection of ketones and aldehydes having an alpha methyl group. The reagents used are iodine and a base which may be $ NaOH $ or $ KOH $ .
Complete step by step solution:
Step $ 1 $ : The base ( $ O{H^ - } $ ) attacks at the $ s{p^2} $ hybridized electrophilic carbon of acetone which breaks the pie bond between carbon and oxygen developing a negative charge on oxygen atom.
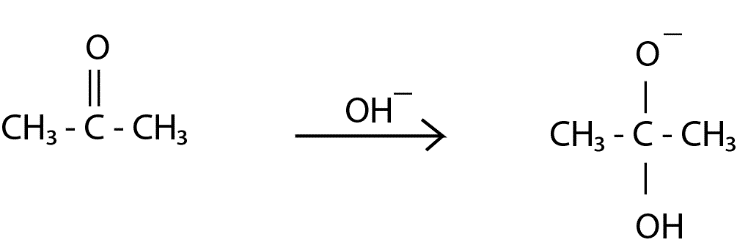
Step $ 2 $ : Again, the base ( $ O{H^ - } $ ) attacks but this time it takes $ H $ from the $ C{H_3} $ moiety and forms water.
Simultaneously, $ {I_2} $ reacts with the formed molecule where $ {I^ + } $ gets attached to the $ C{H_2}^ - $ formed and $ {I^ - } $ ion remains in the solution.
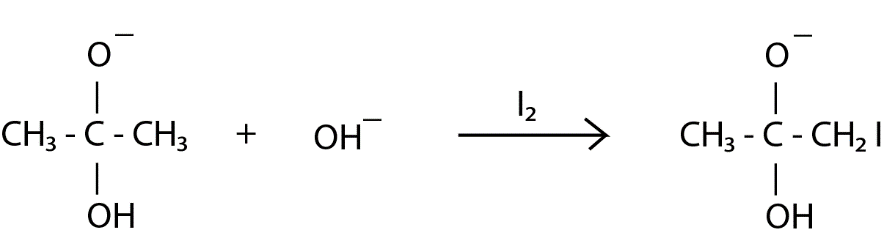
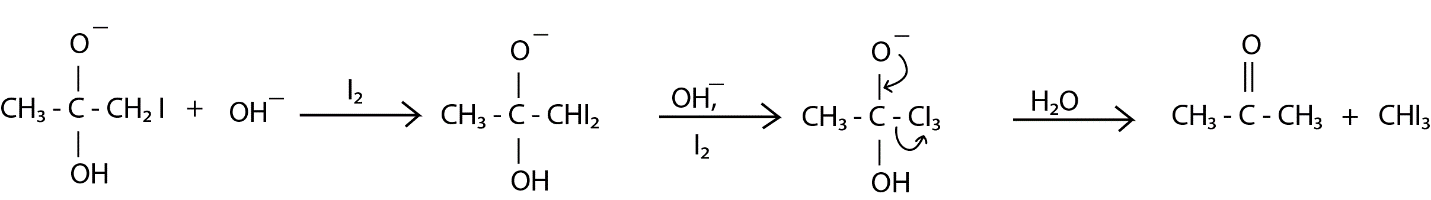
This step is repeated for the remaining two $ H $ present in methyl moiety. Now, oxygen donated its lone pair forming a pie bond with carbon and $ C{I_3}^ - $ goes away forming acetic acid. From the molecules of $ {H_2}O $ formed, one molecule reacts with $ C{I_3}^ - $ and $ CH{I_3} $ (iodoform) is formed which is light yellow color precipitate.
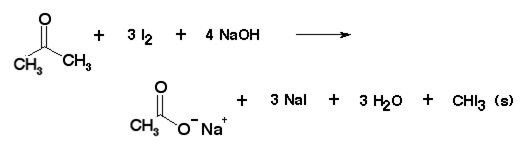
So, the complete balanced reaction combining all the steps is

If $ NaOH $ is taken as the base for the above reaction, then the reaction would be,
Additional information:
When iodine and sodium hydroxide are added to a compound that contains either a methyl ketone or a secondary alcohol with a methyl group in the alpha position, a pale yellow precipitate of iodoform or triiodomethane is formed. It can be used to identify aldehydes and ketones. The only aldehyde which gives this test is acetaldehyde since it is the only aldehyde which costs $ C{H_3}CO $ group.
Note:
The test can also be used to identify alcohols; if the alcohol is secondary alcohol then it gives no results as it cannot be oxidized. If the alcohol is primary alcohol, then it must be ethanol (as it can be oxidized to ethanal, which is the only aldehyde that gives a positive result with the iodoform test). All secondary alcohols give the positive result as they are oxidized to ketones.
Complete step by step solution:
Step $ 1 $ : The base ( $ O{H^ - } $ ) attacks at the $ s{p^2} $ hybridized electrophilic carbon of acetone which breaks the pie bond between carbon and oxygen developing a negative charge on oxygen atom.

Step $ 2 $ : Again, the base ( $ O{H^ - } $ ) attacks but this time it takes $ H $ from the $ C{H_3} $ moiety and forms water.
Simultaneously, $ {I_2} $ reacts with the formed molecule where $ {I^ + } $ gets attached to the $ C{H_2}^ - $ formed and $ {I^ - } $ ion remains in the solution.
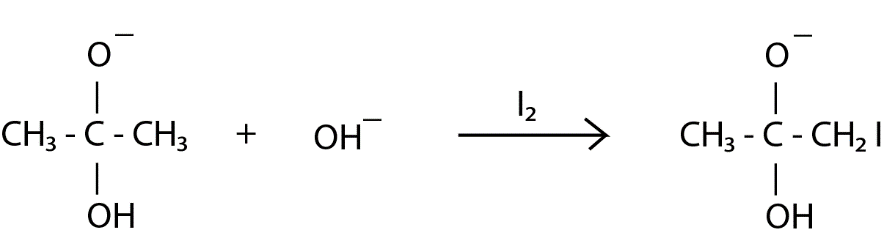
This step is repeated for the remaining two $ H $ present in methyl moiety. Now, oxygen donated its lone pair forming a pie bond with carbon and $ C{I_3}^ - $ goes away forming acetic acid. From the molecules of $ {H_2}O $ formed, one molecule reacts with $ C{I_3}^ - $ and $ CH{I_3} $ (iodoform) is formed which is light yellow color precipitate.

So, the complete balanced reaction combining all the steps is

If $ NaOH $ is taken as the base for the above reaction, then the reaction would be,

Additional information:
When iodine and sodium hydroxide are added to a compound that contains either a methyl ketone or a secondary alcohol with a methyl group in the alpha position, a pale yellow precipitate of iodoform or triiodomethane is formed. It can be used to identify aldehydes and ketones. The only aldehyde which gives this test is acetaldehyde since it is the only aldehyde which costs $ C{H_3}CO $ group.
Note:
The test can also be used to identify alcohols; if the alcohol is secondary alcohol then it gives no results as it cannot be oxidized. If the alcohol is primary alcohol, then it must be ethanol (as it can be oxidized to ethanal, which is the only aldehyde that gives a positive result with the iodoform test). All secondary alcohols give the positive result as they are oxidized to ketones.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




