
The Kolbe’s electrolysis of potassium succinate gives $C{O_2}$ and _____
A.Ethene
B.Ethane
C.Methane
D.Methanol
Answer
522.3k+ views
Hint: We have to know that in Kolbe’s electrolysis, the dicarboxylic molecules undergo decarboxylation first and products alkenes are formed by dimerization. We have to know that a radical intermediate forms after decarboxylation, the intermediate gets combined, and alkene is formed as a product.
Complete answer:
We have to know that hydrocarbons which are useful substances are produced by Kolbe’s electrolysis. With the help of liquid ammonia, Kolbe electrolysis could be modified. In anode, free radicals are formed and once the free radicals are formed at anode, they go through dimerization and alkenes are formed as a product.
We can draw the structure of potassium succinate as,

Symmetrical hydrocarbons are formed by Kolbe’s electrolysis. We can write the chemical equation for Kolbe’s electrolysis for potassium succinate as,
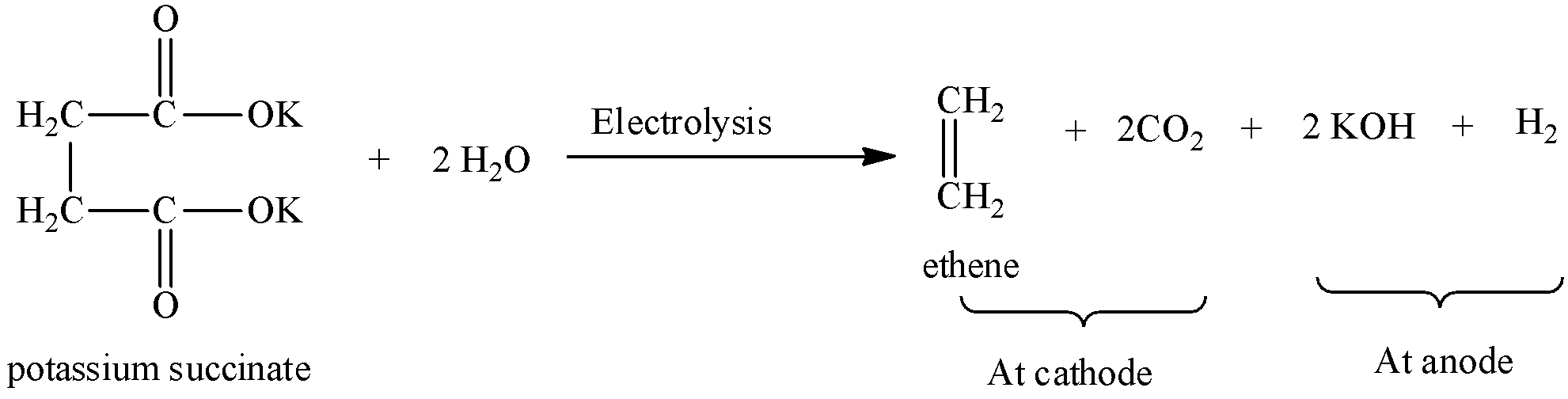
At cathode, carbon dioxide and ethene is formed by decarboxylation of potassium succinate.
At anode, potassium hydroxide and hydrogen gas is formed.
So, Kolbe’s electrolysis of potassium succinate results in the formation of ethene and carbon dioxide.
Option (A) is correct.
Note:
We could use Kolbe’s electrolysis to form polymers that have low molecular weight and also polystyrene could be prepared by Kolbe’s electrolysis. We have to know that for any organic molecule that contains two carboxylic acids on side by side carbon, they are not unstable and to provide stabilization, alkenes are formed as a product. We have to know that the number of carbon dioxide molecules that are given out in Kolbe’s electrolysis is the carboxylic acid functional group found in the molecule.
Complete answer:
We have to know that hydrocarbons which are useful substances are produced by Kolbe’s electrolysis. With the help of liquid ammonia, Kolbe electrolysis could be modified. In anode, free radicals are formed and once the free radicals are formed at anode, they go through dimerization and alkenes are formed as a product.
We can draw the structure of potassium succinate as,

Symmetrical hydrocarbons are formed by Kolbe’s electrolysis. We can write the chemical equation for Kolbe’s electrolysis for potassium succinate as,

At cathode, carbon dioxide and ethene is formed by decarboxylation of potassium succinate.
At anode, potassium hydroxide and hydrogen gas is formed.
So, Kolbe’s electrolysis of potassium succinate results in the formation of ethene and carbon dioxide.
Option (A) is correct.
Note:
We could use Kolbe’s electrolysis to form polymers that have low molecular weight and also polystyrene could be prepared by Kolbe’s electrolysis. We have to know that for any organic molecule that contains two carboxylic acids on side by side carbon, they are not unstable and to provide stabilization, alkenes are formed as a product. We have to know that the number of carbon dioxide molecules that are given out in Kolbe’s electrolysis is the carboxylic acid functional group found in the molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




