
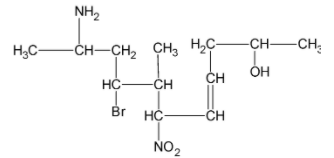
The IUPAC name of the structure is:
A. 5-amino-3-bromo-5 methyl-6-nitroundec-3-en-2-ol
B. 9-amino-3-bromo-7-methyl-4-nitroundec-2-en-2-ol
C. 10-amino-8-bromo-7-methyl-6-nitroundec-4-en-2-ol
D. 2-amino-6-bromo-5-methyl-4-nitroundec-2-en-2-ol

Answer
548.7k+ views
Hint:
To answer this question, you should recall the rules for IUPAC nomenclature of polyfunctional organic compounds. The order of preference for choice of the principal functional group is:
$ - COOH > - S{O_3}H > - {(CO)_2}O > - COOR > - COX > - CON{H_2} > - CN$$ > - CHO > \rangle C = O > - OH > - SH > - N{H_2} > - OR > \rangle C = C\langle > - C \equiv C - $
Complete step by step solution:
The functional groups present in the compound are amino group, bromo group, hydroxyl group, nitro group and alkene group.
From the preference order, we can see that among the functional groups present in the compound, hydroxyl group is of the highest priority to be chosen as the principal functional group.
The functional groups placed above in the order of preference decides suffix names.
Preference order: Hydroxyl > Amino > Alkene > Nitro
Halo groups and methyl groups are always written as substituents in polyfunctional organic compounds.
Next, we move on to selection of the principal chain. The principal chain must contain the principal function group, maximum number of secondary functional groups and multiple bonds.
The principal chain contains 11 carbon atoms with a double bond at fourth carbon and hydroxyl group at second carbon atom.
Hence the name of the principal chain is undec-4-en-2-ol.
The prefixes for secondary functional groups must be written in alphabetical order before the word root.
The amino group is present at the 10th carbon atom, bromo group at 8th carbon atom, methyl group at 7th carbon and nitro group at 6th carbon atom.
Taking all the above points in consideration we can write the IUPAC name of the compound as,
10-amino-8-bromo-7-methyl-6-nitroundec-4-en-2-ol.
Thus, the correct option is C.
Note:
It should be taken into note that the secondary suffix should be added to the primary suffix by dropping its terminal ‘e ‘.
In the question, the primary suffix has as -ene (for alkene) and the secondary suffix is -ol (for alcohol). Hence, the suffixes are written together as -en-ol.
To answer this question, you should recall the rules for IUPAC nomenclature of polyfunctional organic compounds. The order of preference for choice of the principal functional group is:
$ - COOH > - S{O_3}H > - {(CO)_2}O > - COOR > - COX > - CON{H_2} > - CN$$ > - CHO > \rangle C = O > - OH > - SH > - N{H_2} > - OR > \rangle C = C\langle > - C \equiv C - $
Complete step by step solution:
The functional groups present in the compound are amino group, bromo group, hydroxyl group, nitro group and alkene group.
From the preference order, we can see that among the functional groups present in the compound, hydroxyl group is of the highest priority to be chosen as the principal functional group.
The functional groups placed above in the order of preference decides suffix names.
Preference order: Hydroxyl > Amino > Alkene > Nitro
Halo groups and methyl groups are always written as substituents in polyfunctional organic compounds.
Next, we move on to selection of the principal chain. The principal chain must contain the principal function group, maximum number of secondary functional groups and multiple bonds.
The principal chain contains 11 carbon atoms with a double bond at fourth carbon and hydroxyl group at second carbon atom.
Hence the name of the principal chain is undec-4-en-2-ol.
The prefixes for secondary functional groups must be written in alphabetical order before the word root.
The amino group is present at the 10th carbon atom, bromo group at 8th carbon atom, methyl group at 7th carbon and nitro group at 6th carbon atom.
Taking all the above points in consideration we can write the IUPAC name of the compound as,
10-amino-8-bromo-7-methyl-6-nitroundec-4-en-2-ol.
Thus, the correct option is C.
Note:
It should be taken into note that the secondary suffix should be added to the primary suffix by dropping its terminal ‘e ‘.
In the question, the primary suffix has as -ene (for alkene) and the secondary suffix is -ol (for alcohol). Hence, the suffixes are written together as -en-ol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




