
The IUPAC name of aniline is:
A. phenylamine
B. aminobenzene
C. benzylamine
D. benzenamine
Answer
578.4k+ views
Hint: The phenyl ring is a cyclic ring which is viewed as a benzene ring having formula ${C_6}{H_6}$ with one hydrogen atom less $({C_6}{H_6} - H)$ where the hydrogen atom is replaced by other compounds. The molecular formula of the phenyl group is ${C_6}{H_5}$.
Complete step by step answer:
Aniline is a compound carrying two groups, one is a phenyl ring and the other is an amino group. The amino group is directly attached to the phenyl ring.
Preparation of aniline
When benzene is heated with concentrated sulphuric acid and concentrated nitric acid at $40^\circ C$, nitrobenzene is formed. This reaction is known as nitration. On reacting nitrobenzene with concentrated hydrochloric acid and excess sodium hydroxide in presence of the tin catalyst, aniline is formed.
The reaction is shown below.
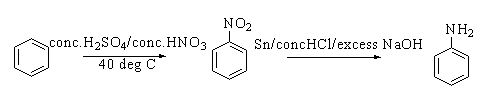
The chemical structure of aniline is shown below.
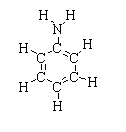
Aniline is commonly known as aminobenzene. The chemical formula of aniline is ${C_6}{H_5}N{H_2}$or ${C_6}{H_7}N$. Aniline consists of six carbon atoms, seven hydrogen atoms, and one nitrogen atom.
As carbon is the chemical formula, aniline is considered as an organic compound. The phenyl is a hexagonal ring with an alternate double bond and the amino group consists of one nitrogen atom joined with two hydrogen atoms. The resulting compound is an aromatic amine.
The IUPAC name of aniline is phenylamine.
Therefore, the correct option is A.
Note:
The phenyl ring is closely connected with the benzene. The alternative name for aniline is given as benzene amine because of the structure. In benzylamine, one methyl group is added and thus the molecular formula of benzylamine is ${C_6}{H_5}C{H_2}N{H_2}$.
Complete step by step answer:
Aniline is a compound carrying two groups, one is a phenyl ring and the other is an amino group. The amino group is directly attached to the phenyl ring.
Preparation of aniline
When benzene is heated with concentrated sulphuric acid and concentrated nitric acid at $40^\circ C$, nitrobenzene is formed. This reaction is known as nitration. On reacting nitrobenzene with concentrated hydrochloric acid and excess sodium hydroxide in presence of the tin catalyst, aniline is formed.
The reaction is shown below.
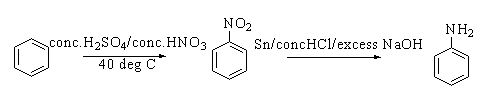
The chemical structure of aniline is shown below.

Aniline is commonly known as aminobenzene. The chemical formula of aniline is ${C_6}{H_5}N{H_2}$or ${C_6}{H_7}N$. Aniline consists of six carbon atoms, seven hydrogen atoms, and one nitrogen atom.
As carbon is the chemical formula, aniline is considered as an organic compound. The phenyl is a hexagonal ring with an alternate double bond and the amino group consists of one nitrogen atom joined with two hydrogen atoms. The resulting compound is an aromatic amine.
The IUPAC name of aniline is phenylamine.
Therefore, the correct option is A.
Note:
The phenyl ring is closely connected with the benzene. The alternative name for aniline is given as benzene amine because of the structure. In benzylamine, one methyl group is added and thus the molecular formula of benzylamine is ${C_6}{H_5}C{H_2}N{H_2}$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




