
The IUPAC name of an unsymmetrical ether with the molecular formula ${{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}$ is:
(A) Ethoxy propane.
(B) Methoxy ethane.
(C) Ethoxy ethane.
(D) Methoxy propane.
Answer
570k+ views
Hint: We will first draw the structure of all the compounds given in the options and then compare them with the given molecular formula and also see whether it is unsymmetrical or not. Initially we count the number of carbon atoms in the carbon chain.
Complete step by step answer:
The naming of the ether is very simple; we see the substituents group on each side of the oxygen. The more complex substituent name becomes the root word and the less complex part is named as alkoxy.
Now we will see the structure of compounds of every option:
(A) Ethoxy propane
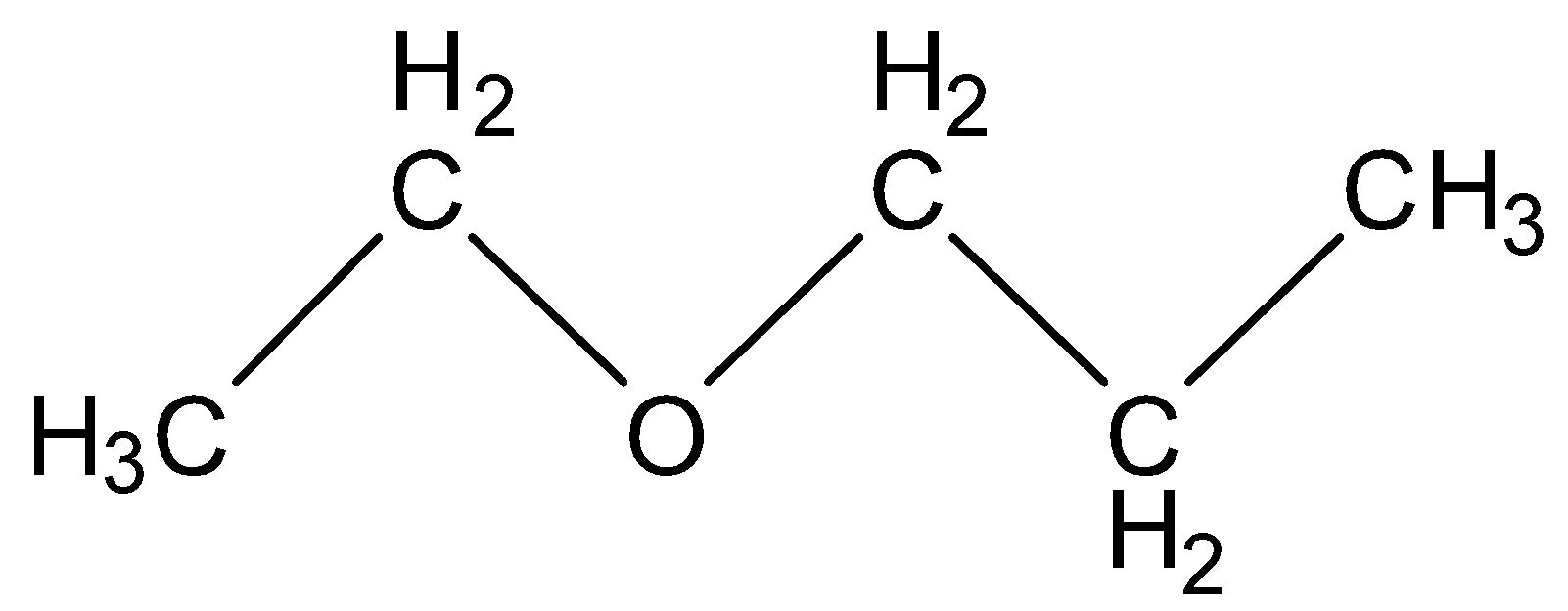
In this structure there are five carbons and twelve hydrogen present which is not equal to the molecular formula given in question above. So, it is an incorrect option.
(B) Methoxy ethane
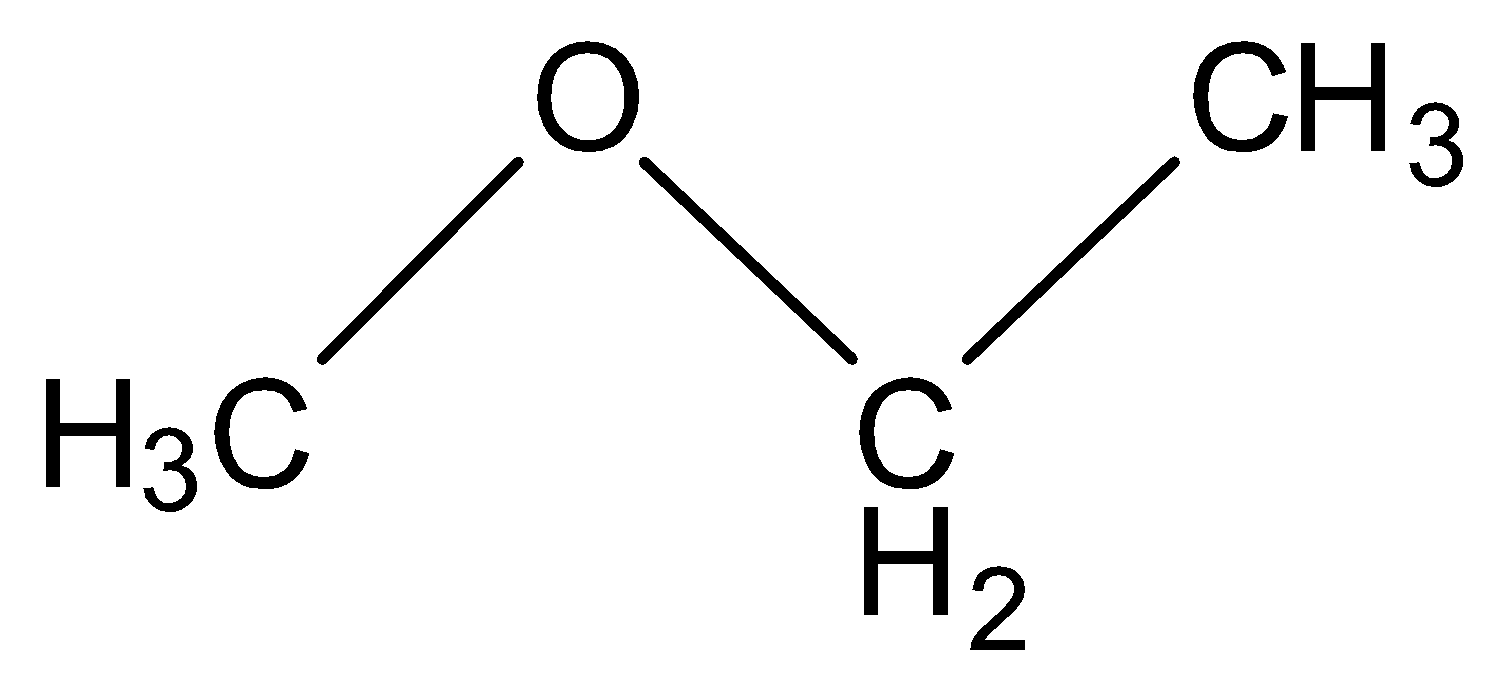
This structure contains three carbon and eight hydrogen which is again not equal to the molecular formula given in the question so this is also an incorrect option.
(C) Ethoxy ethane
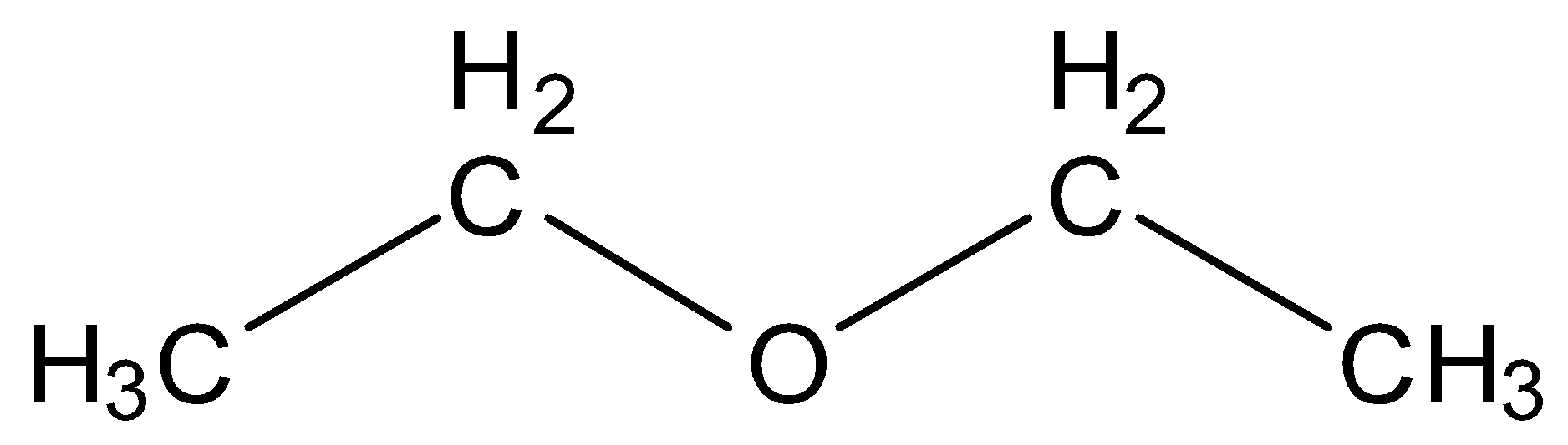
This structure contains four carbon and ten hydrogen which is equal to the molecular formula given in the question but it is still not the correct option as the ether here is symmetrical which is not a condition of answer so incorrect option.
(D) Methoxy propane
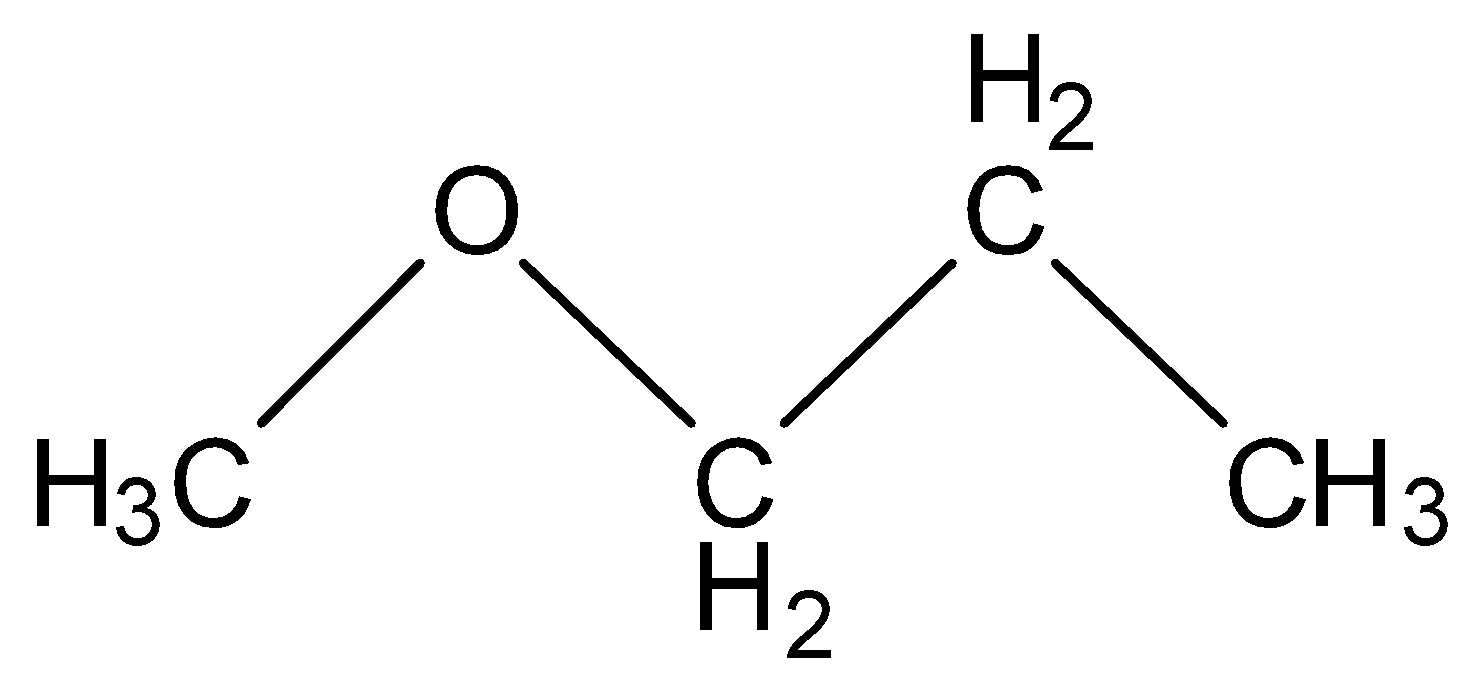
The structure here has ten hydrogens and four carbons and also fulfills the condition of being unsymmetrical so this is the correct option.
So, the correct answer is Option D .
Additional information:
The nomenclature of ether is in the form of alkoxy alkane in which the complex substituent is the root word alkane and less complex substituent is alkoxy. The linkage between carbon oxygen and carbon is bent at an angle of ${{111^\circ }}$ and the bond length of carbon oxygen bond is ${\text{141 pm}}$.
Note: There is a large electronegativity difference between carbon and oxygen so it is polar in nature and the hydrogens attached to carbon ${{\alpha }}$ to oxygen are acidic in nature and can be easily removed.
Complete step by step answer:
The naming of the ether is very simple; we see the substituents group on each side of the oxygen. The more complex substituent name becomes the root word and the less complex part is named as alkoxy.
Now we will see the structure of compounds of every option:
(A) Ethoxy propane

In this structure there are five carbons and twelve hydrogen present which is not equal to the molecular formula given in question above. So, it is an incorrect option.
(B) Methoxy ethane

This structure contains three carbon and eight hydrogen which is again not equal to the molecular formula given in the question so this is also an incorrect option.
(C) Ethoxy ethane

This structure contains four carbon and ten hydrogen which is equal to the molecular formula given in the question but it is still not the correct option as the ether here is symmetrical which is not a condition of answer so incorrect option.
(D) Methoxy propane

The structure here has ten hydrogens and four carbons and also fulfills the condition of being unsymmetrical so this is the correct option.
So, the correct answer is Option D .
Additional information:
The nomenclature of ether is in the form of alkoxy alkane in which the complex substituent is the root word alkane and less complex substituent is alkoxy. The linkage between carbon oxygen and carbon is bent at an angle of ${{111^\circ }}$ and the bond length of carbon oxygen bond is ${\text{141 pm}}$.
Note: There is a large electronegativity difference between carbon and oxygen so it is polar in nature and the hydrogens attached to carbon ${{\alpha }}$ to oxygen are acidic in nature and can be easily removed.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




