
The IUPAC name for the compound is:
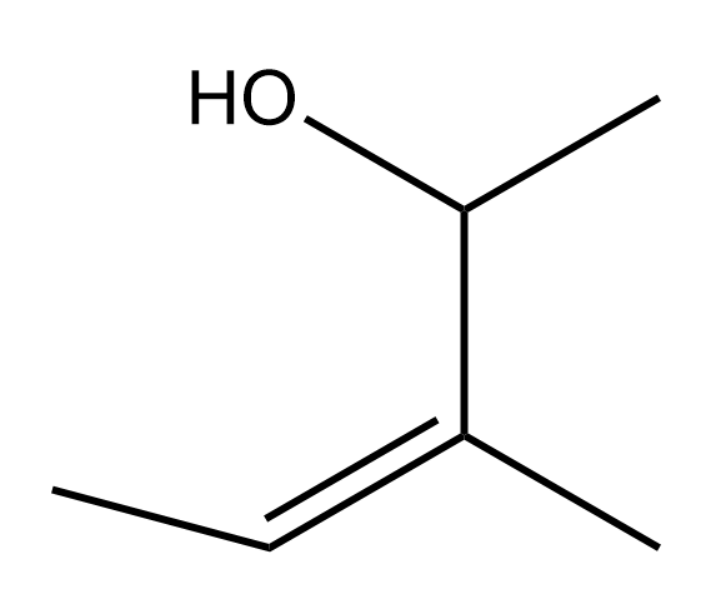
A.3,4-Dimethyl-2-butene-4-ol
B.1,2-Dimethyl-2-butenol
C.3-Methyl pent-3-en-2-ol
D.2,3-Dimethyl-3-pentenol

Answer
501.9k+ views
Hint: To find the IUPAC name of an organic compound always refer to the rules. We need to first find out the longest carbon chain and then find the functional group which should be given preference. That group should have the lowest number and the remaining branches and groups are numbered in that order.
Complete answer:
In this question, we can say that the longest chain will be the chain containing 5 carbons and hence the parent chain can be named as pentane.
Alcohol group is given preference over the double bond according to the IUPAC rules and thus the numbering will be as follows:
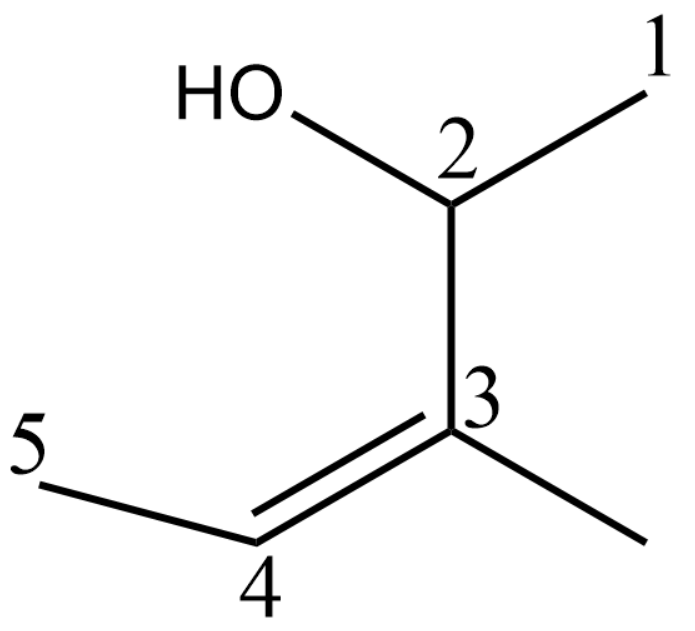
Now we can say that there is one methyl group at the 3rd position and the hydroxyl group is at the 2nd position. The double bond will get a number of 3
Due to the double bond at the third position, we can say that the name becomes pent-3-ene.
Now adding the alcohol group which is at the second position we can expand the name as pent-3-en-2-ol since alcohol groups are given the suffix –ol.
Finally, we can say that the third position has a methyl group and thus the name becomes 3-Methyl pent-3-en-2-ol
Thus the correct option is (C).
Note:
Take care while writing the IUPAC name that the vowels should be removed from the name if it is followed by another vowel. For example, in this question, the answer should be 3-Methyl pent-3-en-2-ol, not 3-Methyl pent-3-ene-2-ol because the “e” of ene and “o” of ol cannot be together. This should be kept in mind while answering naming questions.
Complete answer:
In this question, we can say that the longest chain will be the chain containing 5 carbons and hence the parent chain can be named as pentane.
Alcohol group is given preference over the double bond according to the IUPAC rules and thus the numbering will be as follows:

Now we can say that there is one methyl group at the 3rd position and the hydroxyl group is at the 2nd position. The double bond will get a number of 3
Due to the double bond at the third position, we can say that the name becomes pent-3-ene.
Now adding the alcohol group which is at the second position we can expand the name as pent-3-en-2-ol since alcohol groups are given the suffix –ol.
Finally, we can say that the third position has a methyl group and thus the name becomes 3-Methyl pent-3-en-2-ol
Thus the correct option is (C).
Note:
Take care while writing the IUPAC name that the vowels should be removed from the name if it is followed by another vowel. For example, in this question, the answer should be 3-Methyl pent-3-en-2-ol, not 3-Methyl pent-3-ene-2-ol because the “e” of ene and “o” of ol cannot be together. This should be kept in mind while answering naming questions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




