
The IUPAC name for the compound
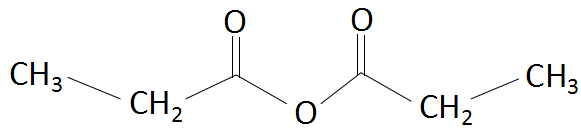 is:
is:
A. Propionic anhydride
B. Dipropionic anhydride
C. Ethoxypropanoic acid
D. Propanoic anhydride

Answer
564.3k+ views
Hint: The name of the organic compounds in organic chemistry is determined by following the certain rules of nomenclature prescribed by the International Union of Pure and Applied Chemistry. The constituent of the name of an organic compound consists of a root word, a primary substituent and a secondary functional group.
Complete step by step answer:
According to the rules of IUPAC nomenclature, there are certain points which must be kept in the mind in order to name any organic compound. As we can see that the parent chain consists of no multiple bonds, so the rules of alkanes should be discussed in this case. The rules for an alkane are as follows:
(i) Find and name the longest continuous carbon chain. (ii) Identify and name groups attached to this chain. (iii) Number the chain consecutively, starting at the end nearest a substituent group. (iv) Designate the location of each substituent group by an appropriate number and name. (v) Assemble the name, listing groups in alphabetical order using the full name (e.g. cyclopropyl before isobutyl). The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing.
Thus, based on the above rules of nomenclature, the name of the given organic compound is propanoic anhydride. The structure is as follows:
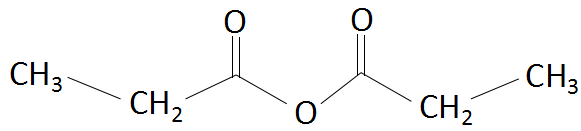
Thus, the correct option is D. Propanoic anhydride.
Note:
Propanoic anhydride is an organic compound with the formula \[{\left( {C{H_3}C{H_2}CO} \right)_2}O\] . This simple acid anhydride is a colorless liquid. It is a widely used reagent in organic synthesis as well as for producing specialty derivatives of cellulose.
Complete step by step answer:
According to the rules of IUPAC nomenclature, there are certain points which must be kept in the mind in order to name any organic compound. As we can see that the parent chain consists of no multiple bonds, so the rules of alkanes should be discussed in this case. The rules for an alkane are as follows:
(i) Find and name the longest continuous carbon chain. (ii) Identify and name groups attached to this chain. (iii) Number the chain consecutively, starting at the end nearest a substituent group. (iv) Designate the location of each substituent group by an appropriate number and name. (v) Assemble the name, listing groups in alphabetical order using the full name (e.g. cyclopropyl before isobutyl). The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing.
Thus, based on the above rules of nomenclature, the name of the given organic compound is propanoic anhydride. The structure is as follows:

Thus, the correct option is D. Propanoic anhydride.
Note:
Propanoic anhydride is an organic compound with the formula \[{\left( {C{H_3}C{H_2}CO} \right)_2}O\] . This simple acid anhydride is a colorless liquid. It is a widely used reagent in organic synthesis as well as for producing specialty derivatives of cellulose.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




