
The internal resistance of the cell is the resistance of
A. Electrolyte not in the cell
B. Electrolyte of the cell
C. Vessel of the cell
D. None of these
Answer
591.6k+ views
Hint: Internal resistance is the resistance of the battery or cell which opposes the current flow within the cell. The construction of the cell is very simple and it is a single unit device and its construction also determines or affects various factors. Here we discuss its construction in brief and its effects.
Complete step-by-step answer:
A cell consists of two electrodes, the positive one and the negative one, dipped in the electrolytic solution. A simple diagram of cell is shown below
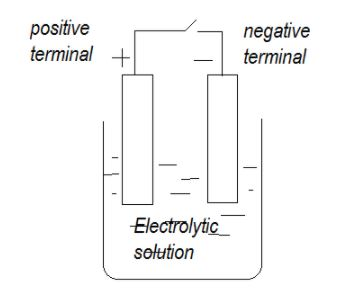
This electrolytic solution is responsible for the current flow as the exchange of charges takes place at the electrodes due to electrolytic solution and when the ions move within the cell they experience the resistive force that is it opposes the current flow, this resistive force is the internal resistance of the cell.
Therefore, the internal resistance of the cell is the resistance of the electrolyte of the cell.
Additional Information:
The internal resistance is not a constant parameter it changes with the time duration of the battery. The new battery or cell will have low internal resistance, with time the internal resistance increases and hence after a while the battery does not conduct or does not work.
The internal resistance of the cell depends on the factors like surface of the electrodes, time duration of the battery, the separation between the electrodes and most importantly the concentration and the type of electrolyte.
Note: Many of us get confused and take the cell and battery as the same but cell and battery are two different things. The group of cells comprises a single battery whereas the cell itself is a single device which converts the chemical energy of the electrolyte into the electrical energy.
Complete step-by-step answer:
A cell consists of two electrodes, the positive one and the negative one, dipped in the electrolytic solution. A simple diagram of cell is shown below

This electrolytic solution is responsible for the current flow as the exchange of charges takes place at the electrodes due to electrolytic solution and when the ions move within the cell they experience the resistive force that is it opposes the current flow, this resistive force is the internal resistance of the cell.
Therefore, the internal resistance of the cell is the resistance of the electrolyte of the cell.
Additional Information:
The internal resistance is not a constant parameter it changes with the time duration of the battery. The new battery or cell will have low internal resistance, with time the internal resistance increases and hence after a while the battery does not conduct or does not work.
The internal resistance of the cell depends on the factors like surface of the electrodes, time duration of the battery, the separation between the electrodes and most importantly the concentration and the type of electrolyte.
Note: Many of us get confused and take the cell and battery as the same but cell and battery are two different things. The group of cells comprises a single battery whereas the cell itself is a single device which converts the chemical energy of the electrolyte into the electrical energy.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




