
The increasing order of $p{{K}_{b}}$ for the following compounds will be:
(a) $N{{H}_{2}}-CH=NH$
(b)
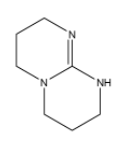
(c) $C{{H}_{3}}-NH-C{{H}_{3}}$

Answer
577.8k+ views
Hint: First we have to find the compound which has the highest basic strength because the compound with highest basic strength will have the highest ${{K}_{b}}$ value and lowest $p{{K}_{b}}$and vice-versa as ${{K}_{b}}=\frac{1}{p{{K}_{b}}}$. Now solve it.
Complete step by step solution:
To find out the compound which has the highest value of $p{{K}_{b}}$, we should know about the basic strength of that compound. The compound which has the highest basic strength , will have the highest ${{K}_{b}}$value and thus, have the lowest value of $p{{K}_{b}}$ and vice-versa. It is so because $p{{K}_{b}}$ is inversely proportional to ${{K}_{b}}$ as;
${{K}_{b}}=\frac{1}{p{{K}_{b}}}$
So, thus one having a higher value of ${{K}_{b}}$, will have a lower value of $p{{K}_{b}}$ and hence, the lowest basic strength.
The basic strength of the compound depends:
1. On the presence of the number of electron donating groups in that very compound.
2. The lone pair or the electron pairs of the donating group should not be in resonance or conjugation.
Now, considering the statement ;
In option (a), there are two $-N{{H}_{2}}$ groups, but the lone pairs on nitrogen atoms are conjugated. So, its basic strength decreases and has lesser ${{K}_{b}}$ value.
In option (b),there are three electron donating nitrogen groups containing lone pair of electrons which are not neither in conjugation nor in resonance with the each other and hence it has the highest basic strength among all and has the largest ${{K}_{b}}$ value.
In option (c), there is one $-N{{H}_{2}}$ group, but the lone pair on the nitrogen atom is in conjugation. So, its basic strength decreases and has lesser ${{K}_{b}}$ value than the option (b) and even from option (a) in which there are two $-N{{H}_{2}}$ groups.
So, the increasing order of the ${{K}_{b}}$ value is as;
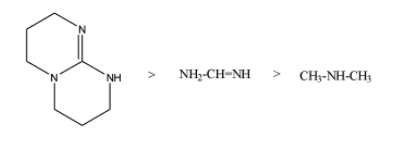
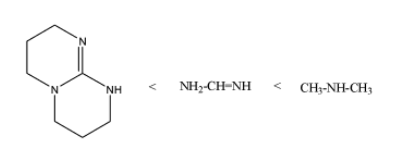
Then, the increasing order of $p{{K}_{b}}$is as;
Note: The electron withdrawing group if attached to the compound, it decreases the basicity as it withdraws the electrons from the compound and its basic strength decreases and hence, have lower ${{K}_{b}}$ values and higher $p{{K}_{b}}$ values.
Complete step by step solution:
To find out the compound which has the highest value of $p{{K}_{b}}$, we should know about the basic strength of that compound. The compound which has the highest basic strength , will have the highest ${{K}_{b}}$value and thus, have the lowest value of $p{{K}_{b}}$ and vice-versa. It is so because $p{{K}_{b}}$ is inversely proportional to ${{K}_{b}}$ as;
${{K}_{b}}=\frac{1}{p{{K}_{b}}}$
So, thus one having a higher value of ${{K}_{b}}$, will have a lower value of $p{{K}_{b}}$ and hence, the lowest basic strength.
The basic strength of the compound depends:
1. On the presence of the number of electron donating groups in that very compound.
2. The lone pair or the electron pairs of the donating group should not be in resonance or conjugation.
Now, considering the statement ;
In option (a), there are two $-N{{H}_{2}}$ groups, but the lone pairs on nitrogen atoms are conjugated. So, its basic strength decreases and has lesser ${{K}_{b}}$ value.
In option (b),there are three electron donating nitrogen groups containing lone pair of electrons which are not neither in conjugation nor in resonance with the each other and hence it has the highest basic strength among all and has the largest ${{K}_{b}}$ value.
In option (c), there is one $-N{{H}_{2}}$ group, but the lone pair on the nitrogen atom is in conjugation. So, its basic strength decreases and has lesser ${{K}_{b}}$ value than the option (b) and even from option (a) in which there are two $-N{{H}_{2}}$ groups.
So, the increasing order of the ${{K}_{b}}$ value is as;

Then, the increasing order of $p{{K}_{b}}$is as;

Note: The electron withdrawing group if attached to the compound, it decreases the basicity as it withdraws the electrons from the compound and its basic strength decreases and hence, have lower ${{K}_{b}}$ values and higher $p{{K}_{b}}$ values.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




