
The incorrect statement among the following are:
A. \[\alpha - D - glucose\;and\,\;\beta - D - glucose\,\] are enantiomers.
B. The pentaacetate of glucose does not react with hydroxylamine.
C. \[\alpha - D - glucose\;and\,\;\beta - D - glucose\,\] are Anomers.
D. Cellulose is straight chain polysaccharide made up of only \[\beta - D - glucose\] units.
Answer
574.2k+ views
Hint: We can solve this question, if we know the difference between the enantiomers and anomers, both contain chiral carbon but one is a mirror image and other is not. One has the structural difference of the only one chiral carbon.
Complete answer
As we know difference between the enantiomers and anomers are enantiomers are those who are mirror images of each other which are non-superimposable and have chiral carbon(carbon with all four different functional group) but anomer are not mirror images but, the difference in structure is due to the attachment of the functional group on the single carbon atom will differ other carbon will have same structure.
Glucose have six carbons as shown in the figure they are attached in a chain form and can also form the cyclic structure without resonance. They have isomeric structure i.e. same number of carbon but different arrangement of the hydroxyl attached to the first carbon if we are considering the glucose isomers \[\alpha - D - glucose\;and\,\;\beta - D - glucose\,\].
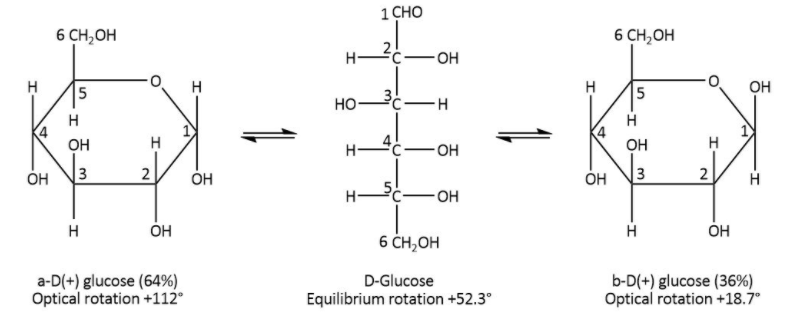
In \[D - glucose\],\[\alpha - D - glucose\;and\,\;\beta - D - glucose\,\] are anomers. Anomers are the isomers which have different structures but have the same molecular formula. The first carbon atom will have hydrogen and hydroxyl groups but the way they are attached is different.
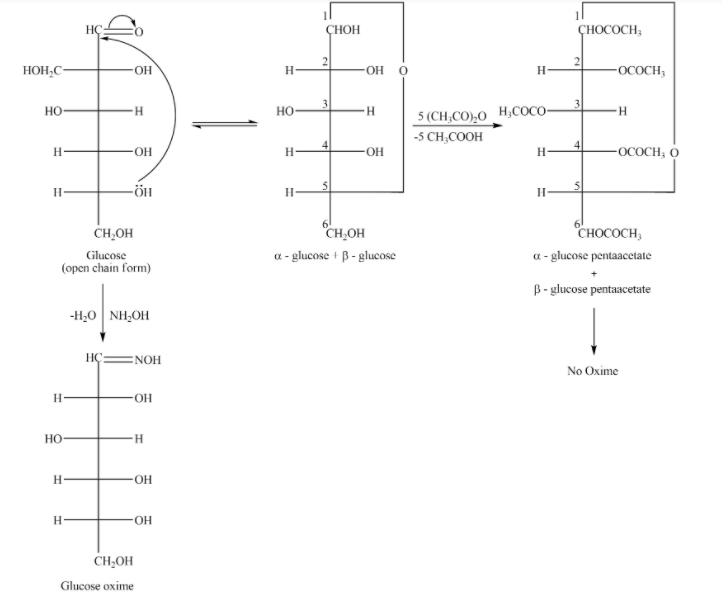
option (B) The pentaacetate of glucose does not react with hydroxylamine because a free carbonyl group is needed to form oxime.
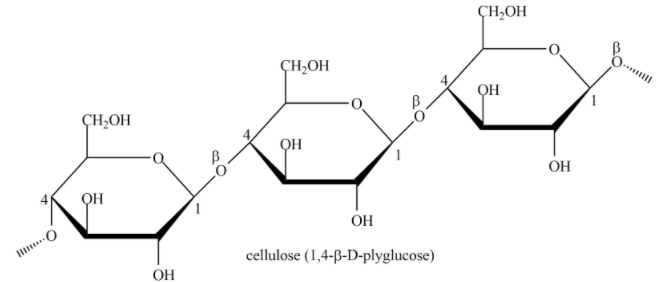
Cellulose is straight chain polysaccharide made up of only \[\beta - D - glucose\] units. Option (D) is true because of this structure.
\[\alpha - D - glucose\;and\,\;\beta - D - glucose\,\] are anomers.
Therefore, the correct answer is Option A. \[\alpha - D - glucose\;and\,\;\beta - D - glucose\,\] are enantiomers is incorrect.
Note:
Here the structure which is different is asked. The definition and understanding of the structure is must. The cyclic and chain structure of glucose is formed and the enantiomers are not possible because cyclic structure cannot be a mirror image of each other.
Complete answer
As we know difference between the enantiomers and anomers are enantiomers are those who are mirror images of each other which are non-superimposable and have chiral carbon(carbon with all four different functional group) but anomer are not mirror images but, the difference in structure is due to the attachment of the functional group on the single carbon atom will differ other carbon will have same structure.
Glucose have six carbons as shown in the figure they are attached in a chain form and can also form the cyclic structure without resonance. They have isomeric structure i.e. same number of carbon but different arrangement of the hydroxyl attached to the first carbon if we are considering the glucose isomers \[\alpha - D - glucose\;and\,\;\beta - D - glucose\,\].

In \[D - glucose\],\[\alpha - D - glucose\;and\,\;\beta - D - glucose\,\] are anomers. Anomers are the isomers which have different structures but have the same molecular formula. The first carbon atom will have hydrogen and hydroxyl groups but the way they are attached is different.

option (B) The pentaacetate of glucose does not react with hydroxylamine because a free carbonyl group is needed to form oxime.
Cellulose is straight chain polysaccharide made up of only \[\beta - D - glucose\] units. Option (D) is true because of this structure.

\[\alpha - D - glucose\;and\,\;\beta - D - glucose\,\] are anomers.
Therefore, the correct answer is Option A. \[\alpha - D - glucose\;and\,\;\beta - D - glucose\,\] are enantiomers is incorrect.
Note:
Here the structure which is different is asked. The definition and understanding of the structure is must. The cyclic and chain structure of glucose is formed and the enantiomers are not possible because cyclic structure cannot be a mirror image of each other.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




