
The hybridization of the central atom $S$ in $S{{O}_{2}}$ is:
A. $s{{p}^{2}}$
B. $s{{p}^{3}}$
C. $sp{{d}^{3}}$
D. ${{d}^{3}}s$
Answer
584.4k+ views
Hint: We can calculate the steric factor for this molecule and then according to this number, we can assign the hybridization to this molecule. Steric factor of any molecule is the sum of the sigma bonds it contains and the number of its lone pairs.
Complete answer:
First, let us see the definition of hybridization.
- Hybridization is the phenomenon of mixing of orbitals of slightly different energies so as to redistribute their energies resulting in the formation of a new set of orbitals of equivalent energies and shape called degenerate orbitals.
- To predict the hybridization of a given molecule, first calculate the steric number ($X$) of the given molecule. The steric Number ($X$) is equal to the sum of the number of sigma bonds around that atom and the number of lone pairs on that atom. This can be represented as:
Steric factor ($X$ ) = number of sigma bonds + number of lone pairs.
- The structure of $S{{O}_{2}}$ is:
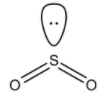
- It is known to you that hybridization for the given steric values is as follows:
\[\begin{align}
& X=2\Rightarrow sp \\
& X=3\Rightarrow s{{p}^{2}} \\
& X=4\Rightarrow s{{p}^{3}} \\
& X=5\Rightarrow s{{p}^{3}}d \\
& X=6\Rightarrow s{{p}^{3}}{{d}^{2}} \\
& X=7\Rightarrow s{{p}^{3}}{{d}^{3}} \\
\end{align}\]
- Determination of hybridization of $S{{O}_{2}}$ :
Number of sigma bond around the Sulphur atom in \[S{{O}_{2}}\] = 2 (one with each oxygen atom)
Sulphur atom has 6 valence electrons, out of which 4 of them are shared with two oxygen atoms in $S{{O}_{2}}$ .
So, Number of lone pairs of electrons on a Sulphur atom in $S{{O}_{2}}=\dfrac{6-4}{2}=1$ .
Hence, steric number ($X$) for $S{{O}_{2}}$ = 2 + 1 = 3 and it is already known to you that for X = 3, hybridization is $s{{p}^{2}}$.
Therefore, from above it can be easily concluded that ‘A. $s{{p}^{2}}$’ is the correct answer to the given question.
Note:
Note that the shape of $S{{O}_{2}}$ molecule is a ‘V’ or ‘Bent’ or ‘Angular’ shape and its molecular geometry is trigonal planar. Also, you should remember that in hybridization the number of hybrid orbitals is equal to the number of the atomic orbitals that get hybridized.
Complete answer:
First, let us see the definition of hybridization.
- Hybridization is the phenomenon of mixing of orbitals of slightly different energies so as to redistribute their energies resulting in the formation of a new set of orbitals of equivalent energies and shape called degenerate orbitals.
- To predict the hybridization of a given molecule, first calculate the steric number ($X$) of the given molecule. The steric Number ($X$) is equal to the sum of the number of sigma bonds around that atom and the number of lone pairs on that atom. This can be represented as:
Steric factor ($X$ ) = number of sigma bonds + number of lone pairs.
- The structure of $S{{O}_{2}}$ is:

- It is known to you that hybridization for the given steric values is as follows:
\[\begin{align}
& X=2\Rightarrow sp \\
& X=3\Rightarrow s{{p}^{2}} \\
& X=4\Rightarrow s{{p}^{3}} \\
& X=5\Rightarrow s{{p}^{3}}d \\
& X=6\Rightarrow s{{p}^{3}}{{d}^{2}} \\
& X=7\Rightarrow s{{p}^{3}}{{d}^{3}} \\
\end{align}\]
- Determination of hybridization of $S{{O}_{2}}$ :
Number of sigma bond around the Sulphur atom in \[S{{O}_{2}}\] = 2 (one with each oxygen atom)
Sulphur atom has 6 valence electrons, out of which 4 of them are shared with two oxygen atoms in $S{{O}_{2}}$ .
So, Number of lone pairs of electrons on a Sulphur atom in $S{{O}_{2}}=\dfrac{6-4}{2}=1$ .
Hence, steric number ($X$) for $S{{O}_{2}}$ = 2 + 1 = 3 and it is already known to you that for X = 3, hybridization is $s{{p}^{2}}$.
Therefore, from above it can be easily concluded that ‘A. $s{{p}^{2}}$’ is the correct answer to the given question.
Note:
Note that the shape of $S{{O}_{2}}$ molecule is a ‘V’ or ‘Bent’ or ‘Angular’ shape and its molecular geometry is trigonal planar. Also, you should remember that in hybridization the number of hybrid orbitals is equal to the number of the atomic orbitals that get hybridized.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




