
The hybridization of \[PC{{l}_{5}}\] molecule is:
(a)- \[s{{p}^{3}}\]
(b)- \[s{{p}^{2}}{{d}^{2}}\]
(c)- \[s{{p}^{3}}d\]
(d)- \[s{{p}^{2}}\]
Answer
595.2k+ views
Hint: When one s, three p and one d-atomic orbitals mix, it forms five \[s{{p}^{3}}d\] hybrid orbitals of equal energy called \[s{{p}^{3}}d\] hybridization.
When one s, three p and two d orbitals mix, it forms six identical hybrid orbitals called \[s{{p}^{3}}{{d}^{2}}\] hybridization.
Similarly, on mixing one s and three p orbitals, three identical hybrid orbitals are formed called \[s{{p}^{3}}\] hybridization.
Complete step by step solution:
The electronic configuration of phosphorus and chlorine is \[[Ne]\,3{{s}^{2}}3{{p}^{3}}\] and \[[Ne]\,3{{s}^{2}}3{{p}^{5}}\]respectively. We can see that the number of valence electrons in the phosphorus atom is 5, here in \[PC{{l}_{5}}\], according to VSEPR theory it will form a single bond with each of the chlorine atoms i.e. Phosphorus atom needs five orbitals to form the five P-Cl bonds.
It has two electrons in its 3s orbital and three in 3p orbitals in the valence shell, so it must use one of its 3d to form the fifth bond, So, one electron from 3s gets excited to the d orbital and they form five hybrid orbitals.
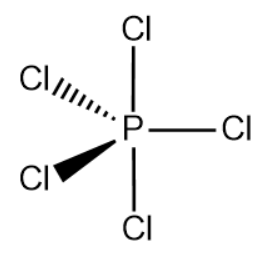
Therefore, the hybridization of \[PC{{l}_{5}}\] id \[s{{p}^{3}}d\].Hence, the correct option is (c).
Additional Information:
Phosphorus pentachloride is a greenish-yellow solid, having a trigonal bipyramid structure. It is readily soluble in water.
Note: Hybridization of a molecule can also be calculated by a formula:
Hybridization = \[\dfrac{1}{2}(V+M-C+A)\]
where, V = valence electrons,
M = monovalent atom linked to central atom,
C = charge on cation,
A = charge on anion.
After getting the numerical value,
2 = \[sp\]
3 = \[s{{p}^{2}}\]
4 = \[s{{p}^{3}}\]
5 = \[s{{p}^{3}}d\]
6 = \[s{{p}^{3}}{{d}^{2}}\]
When one s, three p and two d orbitals mix, it forms six identical hybrid orbitals called \[s{{p}^{3}}{{d}^{2}}\] hybridization.
Similarly, on mixing one s and three p orbitals, three identical hybrid orbitals are formed called \[s{{p}^{3}}\] hybridization.
Complete step by step solution:
The electronic configuration of phosphorus and chlorine is \[[Ne]\,3{{s}^{2}}3{{p}^{3}}\] and \[[Ne]\,3{{s}^{2}}3{{p}^{5}}\]respectively. We can see that the number of valence electrons in the phosphorus atom is 5, here in \[PC{{l}_{5}}\], according to VSEPR theory it will form a single bond with each of the chlorine atoms i.e. Phosphorus atom needs five orbitals to form the five P-Cl bonds.
It has two electrons in its 3s orbital and three in 3p orbitals in the valence shell, so it must use one of its 3d to form the fifth bond, So, one electron from 3s gets excited to the d orbital and they form five hybrid orbitals.

Therefore, the hybridization of \[PC{{l}_{5}}\] id \[s{{p}^{3}}d\].Hence, the correct option is (c).
Additional Information:
Phosphorus pentachloride is a greenish-yellow solid, having a trigonal bipyramid structure. It is readily soluble in water.
Note: Hybridization of a molecule can also be calculated by a formula:
Hybridization = \[\dfrac{1}{2}(V+M-C+A)\]
where, V = valence electrons,
M = monovalent atom linked to central atom,
C = charge on cation,
A = charge on anion.
After getting the numerical value,
2 = \[sp\]
3 = \[s{{p}^{2}}\]
4 = \[s{{p}^{3}}\]
5 = \[s{{p}^{3}}d\]
6 = \[s{{p}^{3}}{{d}^{2}}\]
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




