
The hybridisation of central atom in ${N_3}^ - $, NOCl and ${N_2}O$ respectively are:
(A) sp, $s{p^2}$, sp
(B) sp, sp, $s{p^3}$
(C) $s{p^2}$, sp, sp
(D) $s{p^2}$, $s{p^2}$, sp
Answer
597k+ views
Hint: First see the number of electrons in the outermost orbits of the elements and then count the number of bonding regions and lone pairs. Accordingly see what the hybridisation is. Also identify the correct central atom.
Complete answer:
-Before we start you must know that when we say 2 electron regions it means sp hybridisation, 3 electron regions means $s{p^2}$, 4 electron regions means $s{p^3}$ and so on.
-${N_3}^ - $ : It forms a linear structure. The central N ion forms double bonds with the other 2 N atoms. Thus it has 2 bonding domains and no lone pair of electrons. This gives it a hybridisation of: sp.
So, the hybridisation of the central atom of ${N_3}^ - $ is sp.
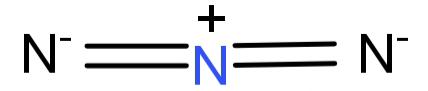
-NOCl: Since in this compound there are 3 elements there is no overall hybridisation and we need to see that individually.
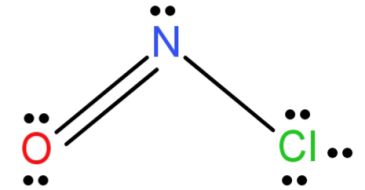 Let’s begin with N. N atom (has 5 electrons in outermost orbit) forms a double bond with oxygen and a single bond with chlorine. It also has a lone pair of electrons left to it. This gives it 3 electron domains and hence its hybridisation is $s{p^2}$.
Let’s begin with N. N atom (has 5 electrons in outermost orbit) forms a double bond with oxygen and a single bond with chlorine. It also has a lone pair of electrons left to it. This gives it 3 electron domains and hence its hybridisation is $s{p^2}$.
For O (it has 6 electrons in outermost orbit): It forms 1 double bond with N atom and is left with 2 lone pairs of electrons. This gives it 3 electron regions. So, its hybridisation is $s{p^2}$.
Now for Cl (has 7 electrons in outermost orbit): it forms only one single bond with the n atom and is left with 3 lone pairs of electrons. This gives it 4 electron domains and thus $s{p^3}$ hybridisation.
Now we need to see which of them is the central atom.
NOCl structure has geometry of trigonal planar and N has $s{p^2}$ hybridisation. N is the central atom because it is less electronegative than O atom and Cl cannot be the central atom because it only forms a single bond.
So, the central atom of NOCl is N and its hybridisation is $s{p^2}$.
-${N_2}O$: Let us first see the hybridisation of all the elements individually.
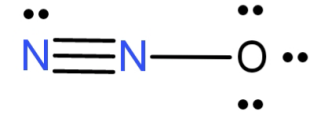
In this both the N atoms have sp hybridisation because the terminal N forms only 1 triple bond with another N atom and has a lone pair of electron and the other n atom forms 1 triple bond with the terminal N atom and a single bond with the O atom. Thus both the N atoms have 2 electron domains and thus sp hybridisation.
The O atom forms a single bond with the central N atom and is left with 3 lone pairs of electrons. Thus it has 4 electron domains and so $s{p^3}$ hybridisation.
In ${N_2}O$ the central atom is the N atom which forms a bond with both N and O atoms. Its hybridisation is sp.
So, the correct option is: (A) sp, $s{p^2}$, sp
Note: Sometimes in such questions we might ignore the lone pairs. So, to avoid this first see the number of electrons in the outermost orbit and after counting the bonds just tally if all the electrons have been counted or not.
Also, remember that a triple bond is counted as 1 electron region only and not 3 electron regions, the same thing goes for a double bond also.
Complete answer:
-Before we start you must know that when we say 2 electron regions it means sp hybridisation, 3 electron regions means $s{p^2}$, 4 electron regions means $s{p^3}$ and so on.
-${N_3}^ - $ : It forms a linear structure. The central N ion forms double bonds with the other 2 N atoms. Thus it has 2 bonding domains and no lone pair of electrons. This gives it a hybridisation of: sp.
So, the hybridisation of the central atom of ${N_3}^ - $ is sp.
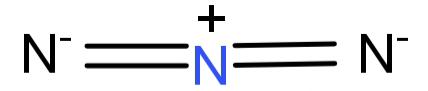
-NOCl: Since in this compound there are 3 elements there is no overall hybridisation and we need to see that individually.

For O (it has 6 electrons in outermost orbit): It forms 1 double bond with N atom and is left with 2 lone pairs of electrons. This gives it 3 electron regions. So, its hybridisation is $s{p^2}$.
Now for Cl (has 7 electrons in outermost orbit): it forms only one single bond with the n atom and is left with 3 lone pairs of electrons. This gives it 4 electron domains and thus $s{p^3}$ hybridisation.
Now we need to see which of them is the central atom.
NOCl structure has geometry of trigonal planar and N has $s{p^2}$ hybridisation. N is the central atom because it is less electronegative than O atom and Cl cannot be the central atom because it only forms a single bond.
So, the central atom of NOCl is N and its hybridisation is $s{p^2}$.
-${N_2}O$: Let us first see the hybridisation of all the elements individually.

In this both the N atoms have sp hybridisation because the terminal N forms only 1 triple bond with another N atom and has a lone pair of electron and the other n atom forms 1 triple bond with the terminal N atom and a single bond with the O atom. Thus both the N atoms have 2 electron domains and thus sp hybridisation.
The O atom forms a single bond with the central N atom and is left with 3 lone pairs of electrons. Thus it has 4 electron domains and so $s{p^3}$ hybridisation.
In ${N_2}O$ the central atom is the N atom which forms a bond with both N and O atoms. Its hybridisation is sp.
So, the correct option is: (A) sp, $s{p^2}$, sp
Note: Sometimes in such questions we might ignore the lone pairs. So, to avoid this first see the number of electrons in the outermost orbit and after counting the bonds just tally if all the electrons have been counted or not.
Also, remember that a triple bond is counted as 1 electron region only and not 3 electron regions, the same thing goes for a double bond also.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




