
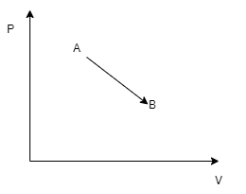
The given indicator diagram shows the variation of pressure with volume, when a thermodynamic system is taken from state A to state B. Identify what happens during the process?
a.The System is cooled
b.The System is Heated
c.The System heated first and then cooled
d.The System cooled first and then heated

Answer
543.6k+ views
Hint: Apply gas law equations with respect to origin points and the given A and B points. Extend the graph so that it touches the poles. Now, using the equation identify the relation between temperature and volume. Conclude using the relation
Complete answer:
From the diagram , we can deduce that A and B are processes of a gas. Let us assume that the gas state given on the diagram is ideal gas. If a gas is said to be ideal and is identified to have 2 states of existence then , we can use the relation,
\[\dfrac{{{P_1}{V_1}}}{{{T_1}}} = \dfrac{{{P_2}{V_2}}}{{{T_2}}}\]
Here, we don’t know the temperature \[{T_1}\] and \[{T_2}\] at position A and B respectively. We apply the gas laws we know for the particular gas and identify the pattern with respect to pressure and volume.
According to Charles law , which states that for a given gas mass, the volume of the gas is directly proportional to the absolute temperature, provided the pressure is constant.
Which means that
\[\dfrac{{{V_A}}}{{{T_A}}} = \dfrac{{{V_B}}}{{{T_B}}}\]
Now, rearranging with like terms on one side,
\[\dfrac{{{V_A}}}{{{V_B}}} = \dfrac{{{T_A}}}{{{T_B}}}\]
This means that greater than value of V, smaller the value of T. In our diagram, V increases in State B. This means that the temperature at point B is lower than point A.
Hence temperature at point A is higher than point B. From (0,0) ,the gas is heated to temperature \[{T_A}\], so that it attains a pressure \[{P_A}\] and Volume \[{V_A}\] and then cools down to \[{T_B}\].
Thus , Option(c) is the right answer for the given question.
Note:
We can also use Boyle’s law to arrive at the given conclusion using pressure as a factor instead of volume.
Complete answer:
From the diagram , we can deduce that A and B are processes of a gas. Let us assume that the gas state given on the diagram is ideal gas. If a gas is said to be ideal and is identified to have 2 states of existence then , we can use the relation,
\[\dfrac{{{P_1}{V_1}}}{{{T_1}}} = \dfrac{{{P_2}{V_2}}}{{{T_2}}}\]
Here, we don’t know the temperature \[{T_1}\] and \[{T_2}\] at position A and B respectively. We apply the gas laws we know for the particular gas and identify the pattern with respect to pressure and volume.
According to Charles law , which states that for a given gas mass, the volume of the gas is directly proportional to the absolute temperature, provided the pressure is constant.
Which means that
\[\dfrac{{{V_A}}}{{{T_A}}} = \dfrac{{{V_B}}}{{{T_B}}}\]
Now, rearranging with like terms on one side,
\[\dfrac{{{V_A}}}{{{V_B}}} = \dfrac{{{T_A}}}{{{T_B}}}\]
This means that greater than value of V, smaller the value of T. In our diagram, V increases in State B. This means that the temperature at point B is lower than point A.
Hence temperature at point A is higher than point B. From (0,0) ,the gas is heated to temperature \[{T_A}\], so that it attains a pressure \[{P_A}\] and Volume \[{V_A}\] and then cools down to \[{T_B}\].
Thus , Option(c) is the right answer for the given question.
Note:
We can also use Boyle’s law to arrive at the given conclusion using pressure as a factor instead of volume.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




