
The function of \[ZnC{{l}_{2}}\] in Lucas test for alcohols is:
a.) To act as an acid catalyst and react with HCl to form \[{{H}_{2}}ZnC{{l}_{4}}\]
b.) To act as a base catalyst and react with NaOH to form \[N{{a}_{2}}Zn{{(OH)}_{4}}\]
c.) To act as an amphoteric catalyst
d.) To act as a neutral catalyst
Answer
520.2k+ views
Hint: Lucas reagent is a mixture of HCl and \[ZnC{{l}_{2}}\]. Lucas reagent is used to test the presence of alcohol in the given compound. Alcohols in organic compounds react with Lucas reagent and form Alkyl halides as the products.
Complete step by step answer:
\[ZnC{{l}_{2}}\]is a Lewis acid because of the presence of empty d-orbitals on Zinc.
Oxygen in –OH group of alcohol forms a coordinate covalent bond with zinc and oxygen acquires a positive charge and Zinc acquires a negative charge.
Therefore oxygen becomes a good leaving group and then the reaction moves towards the formation of the product.
The formed carbonium ion reacts with chlorine in hydrochloric acid and forms alkyl halide as a product.
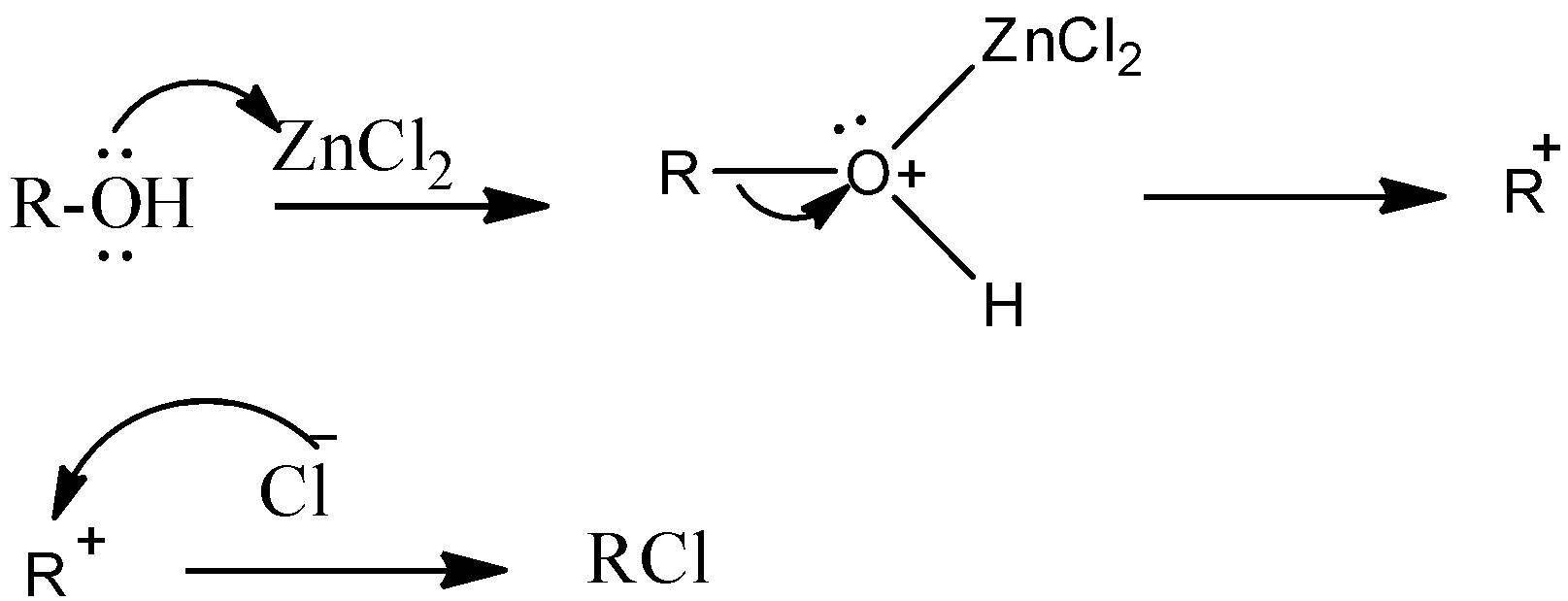
The above reaction can be represented as follows.
\[R-OH+HCl\xrightarrow{ZnC{{l}_{2}}}R-Cl+{{H}_{2}}O\]
In the above reaction \[ZnC{{l}_{2}}\]acts as a catalyst.
\[ZnC{{l}_{2}}\] reacts with the excess of HCl and forms the following complex.
\[ZnC{{l}_{2}}+2HCl\to {{H}_{2}}ZnC{{l}_{4}}\]
Therefore \[ZnC{{l}_{2}}\]acts a catalyst and forms \[{{H}_{2}}ZnC{{l}_{4}}\]by reacting with hydrochloric acid (HCl).
So, the correct answer is “Option A”.
Note: When primary alcohols react with Lucas reagent the solution remains colorless unless it is heated.
Secondary alcohols react with Lucas reagent and form an oily layer in 3-5 minutes.
Tertiary alcohols turn to turbid and form an oily layer immediately by the addition of Lucas reagent.
Complete step by step answer:
\[ZnC{{l}_{2}}\]is a Lewis acid because of the presence of empty d-orbitals on Zinc.
Oxygen in –OH group of alcohol forms a coordinate covalent bond with zinc and oxygen acquires a positive charge and Zinc acquires a negative charge.
Therefore oxygen becomes a good leaving group and then the reaction moves towards the formation of the product.
The formed carbonium ion reacts with chlorine in hydrochloric acid and forms alkyl halide as a product.

The above reaction can be represented as follows.
\[R-OH+HCl\xrightarrow{ZnC{{l}_{2}}}R-Cl+{{H}_{2}}O\]
In the above reaction \[ZnC{{l}_{2}}\]acts as a catalyst.
\[ZnC{{l}_{2}}\] reacts with the excess of HCl and forms the following complex.
\[ZnC{{l}_{2}}+2HCl\to {{H}_{2}}ZnC{{l}_{4}}\]
Therefore \[ZnC{{l}_{2}}\]acts a catalyst and forms \[{{H}_{2}}ZnC{{l}_{4}}\]by reacting with hydrochloric acid (HCl).
So, the correct answer is “Option A”.
Note: When primary alcohols react with Lucas reagent the solution remains colorless unless it is heated.
Secondary alcohols react with Lucas reagent and form an oily layer in 3-5 minutes.
Tertiary alcohols turn to turbid and form an oily layer immediately by the addition of Lucas reagent.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




