
The full name of the compound
(A) $ (2R,3S)-3-Chloro-2-pentanol $
(B) $ (2R,3R)-3-Chloro-2-pentanol $
(C) $ (2R,3R)-3-Chloro-2-pentanol $
(D) $ (2R,3S)-3-Chloro-2-pentanol $

Answer
507.3k+ views
Hint: The compounds with chiral Centre i.e.., the carbon containing four different groups can be named by the nomenclature of R and S before the IUPAC name.
This nomenclature was based on the groups attached to chiral carbon.
Complete answer:
The compounds containing carbon with four different groups can be termed as chiral carbon and the compound can be named by R and S nomenclature.
R stands for Rectus.
S stands for Sinister.
The four groups attached to chiral carbon are given priority or numbered based on atomic masses.
The atoms or groups with higher atomic mass can be given higher priority.
If the direction of high priority groups to low priority groups is in clockwise direction, then the compound can be named as R nomenclature.
If the direction of high priority groups to low priority groups is in anti-clockwise direction, then the compound can be named as S nomenclature.
The least priority should be on the vertical line on the fischer projection in both the above cases.
If the least priority is not on the vertical plane on the Fischer projection, then the configuration can be inverted.
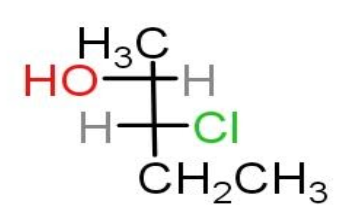
The given compound contains two chiral carbons one is $ {C_2} $ and the other is $ {C_3} $ carbon.
The $ {C_2} $ carbon is S configuration, as the direction of high priority groups to low priority groups is in anti-clockwise direction.
The OH group will be given high priority followed by $ {C_3} $ carbon, methyl group and the least priority is hydrogen atom.
But the least priority is not on the vertical plane on the fischer projection, then the configuration can be inverted to R.
The $ {C_3} $ carbon is S configuration, as the direction of high priority groups to low priority groups is in anti-clockwise direction.
The Cl group will be given high priority followed by $ {C_2} $ carbon, ethyl group and the least priority is hydrogen atom.
But the least priority is not on the vertical plane on the fischer projection, then the configuration can be inverted to R.
Thus, the name of the given compound is $ \left( {2R,3R} \right) - 3 - chloro - 2 - pen\tan ol $ .
Thus, Option B is the correct One.
The remaining options are not given as R configuration.
Note:
While naming the compounds, the priority must be taken according to atomic masses.
The molar mass or atomic mass is the same, thus the atom with higher atomic mass can be preferred firstly. The least priority group must be away from the plane i.e.., below the plane.
This nomenclature was based on the groups attached to chiral carbon.
Complete answer:
The compounds containing carbon with four different groups can be termed as chiral carbon and the compound can be named by R and S nomenclature.
R stands for Rectus.
S stands for Sinister.
The four groups attached to chiral carbon are given priority or numbered based on atomic masses.
The atoms or groups with higher atomic mass can be given higher priority.
If the direction of high priority groups to low priority groups is in clockwise direction, then the compound can be named as R nomenclature.
If the direction of high priority groups to low priority groups is in anti-clockwise direction, then the compound can be named as S nomenclature.
The least priority should be on the vertical line on the fischer projection in both the above cases.
If the least priority is not on the vertical plane on the Fischer projection, then the configuration can be inverted.
The given compound contains two chiral carbons one is $ {C_2} $ and the other is $ {C_3} $ carbon.
The $ {C_2} $ carbon is S configuration, as the direction of high priority groups to low priority groups is in anti-clockwise direction.
The OH group will be given high priority followed by $ {C_3} $ carbon, methyl group and the least priority is hydrogen atom.
But the least priority is not on the vertical plane on the fischer projection, then the configuration can be inverted to R.
The $ {C_3} $ carbon is S configuration, as the direction of high priority groups to low priority groups is in anti-clockwise direction.
The Cl group will be given high priority followed by $ {C_2} $ carbon, ethyl group and the least priority is hydrogen atom.
But the least priority is not on the vertical plane on the fischer projection, then the configuration can be inverted to R.
Thus, the name of the given compound is $ \left( {2R,3R} \right) - 3 - chloro - 2 - pen\tan ol $ .
Thus, Option B is the correct One.
The remaining options are not given as R configuration.
Note:
While naming the compounds, the priority must be taken according to atomic masses.
The molar mass or atomic mass is the same, thus the atom with higher atomic mass can be preferred firstly. The least priority group must be away from the plane i.e.., below the plane.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




