
The following two compounds are__________.
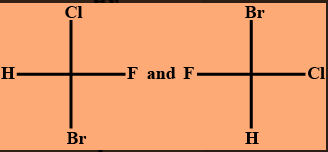
A) enantiomers
B) diastereomers
C) identical
D) epimers

Answer
564.9k+ views
Hint:To solve this question, we must first understand the molecular properties of isomers. Then we need to assess the properties which will help us to conclude the type of isomer and then only we can have our correct answer.
Complete answer:
Before we move forward with the solution of this given question, let us first understand some basic concepts:
Isomerism is the phenomenon in which more than one compounds have the same chemical formula but different chemical structures. Chemical compounds that have identical chemical formulas but differ in properties and the arrangement of atoms in the molecule are called isomers.
And also we must go through the options one by one and then check it with the given question, that it satisfies the question or not:
Enantiomers: Enantiomers are stereoisomers that are non-superimposable mirror images of one another. Whenever a molecule contains a single atom which is tetrahedrally bound to four different substituents, then two enantiomers are possible. It is however important that the four substituents are different to one another as if any two of them are identical then the structure would become superimposable on its mirror image and so achiral. And hence, by verifying the question against our very first option, we can observe that it satisfies the given question. So we got our answer and we need not to check for other options.
Hence, the correct answer option (A).
Note:Whenever a molecule contains a single atom which is tetrahedrally bound to four different substituents, then two enantiomers are possible. It is however important that the four substituents are different to one another as if any two of them are identical then the structure would become superimposable on its mirror image and so achiral.
Complete answer:
Before we move forward with the solution of this given question, let us first understand some basic concepts:
Isomerism is the phenomenon in which more than one compounds have the same chemical formula but different chemical structures. Chemical compounds that have identical chemical formulas but differ in properties and the arrangement of atoms in the molecule are called isomers.
And also we must go through the options one by one and then check it with the given question, that it satisfies the question or not:
Enantiomers: Enantiomers are stereoisomers that are non-superimposable mirror images of one another. Whenever a molecule contains a single atom which is tetrahedrally bound to four different substituents, then two enantiomers are possible. It is however important that the four substituents are different to one another as if any two of them are identical then the structure would become superimposable on its mirror image and so achiral. And hence, by verifying the question against our very first option, we can observe that it satisfies the given question. So we got our answer and we need not to check for other options.
Hence, the correct answer option (A).
Note:Whenever a molecule contains a single atom which is tetrahedrally bound to four different substituents, then two enantiomers are possible. It is however important that the four substituents are different to one another as if any two of them are identical then the structure would become superimposable on its mirror image and so achiral.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




