
The following molecule can be analyzed using a mechanism that involves which one of the following hydrogen atoms:
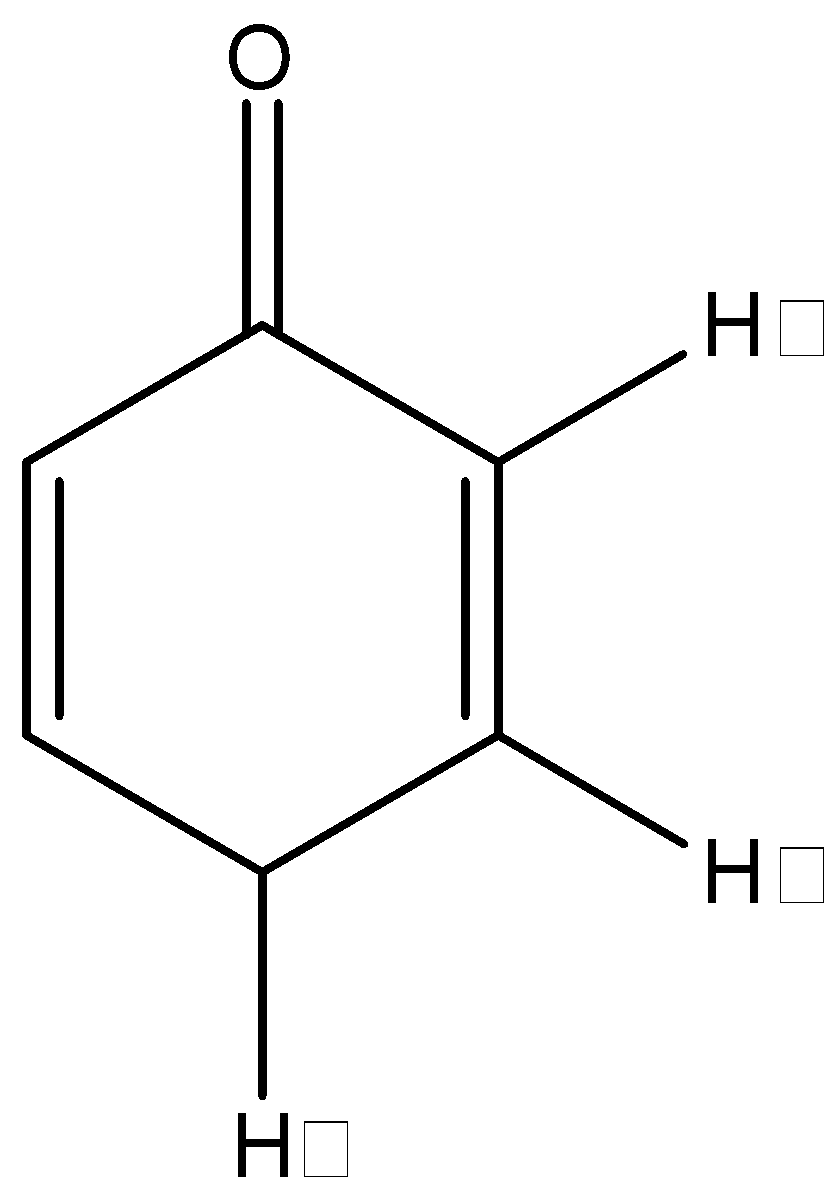
A. ${{H}_{\alpha }}$
B. ${{H}_{\beta }}$
C. ${{H}_{\gamma }}$
D. cannot be enolized

Answer
577.5k+ views
Hint: Recall the condition about the $\alpha $- hydrogen atoms and $s{{p}^{3}}$ hybridized carbons and their presence that is necessary for the keto-enol tautomerization to occur. Think about the deviations from this rule.
Complete answer:
We have always known that the presence of an $s{{p}^{3}}$ hybridized $\alpha $- carbon atom that has an $\alpha $- hydrogen atom attached to it is necessary for the keto enol tautomerization. An $\alpha $- hydrogen atom is an atom that is attached to the $\alpha $- carbon atom which is a direct neighbour of the carbonyl carbon atom or a direct neighbour of any functional group concerned. In the keto-enol tautomerization, the $\alpha $- hydrogen attaches itself to the oxygen atom in the ketone and a double bond is formed between the carbonyl carbon and the $\alpha $- carbon atom. Hence, if an $\alpha $- hydrogen is not present, this tautomerization will not occur.
In this problem, we can see that we have an $\alpha $- hydrogen, but it is not attached to an $s{{p}^{3}}$ hybridized carbon, so tautomerization should not be possible. But there is an exception to this rule that states that if a $\gamma $- hydrogen is attached to an $s{{p}^{3}}$ hybridized carbon, then the tautomerization can occur, and this $\gamma $- hydrogen will take the role of the $\alpha $- hydrogen.
In this problem, we can see that we do in fact have a $\gamma $- hydrogen that is attached to an $s{{p}^{3}}$ hybridized carbon. So, this compound will show keto-enol tautomerization using the $\gamma $- hydrogen. The product after the tautomerization will be:

Hence, the correct answer to this question is ‘C. ${{H}_{\gamma }}$’
Note:
Note that there are actually 2 $\gamma $- hydrogen atoms present on the para-carbon atom and any one of them can be used during the tautomerization. It is just due to convenience’s sake that only one of the two is denoted. Also note that the position of the double bonds change.
Complete answer:
We have always known that the presence of an $s{{p}^{3}}$ hybridized $\alpha $- carbon atom that has an $\alpha $- hydrogen atom attached to it is necessary for the keto enol tautomerization. An $\alpha $- hydrogen atom is an atom that is attached to the $\alpha $- carbon atom which is a direct neighbour of the carbonyl carbon atom or a direct neighbour of any functional group concerned. In the keto-enol tautomerization, the $\alpha $- hydrogen attaches itself to the oxygen atom in the ketone and a double bond is formed between the carbonyl carbon and the $\alpha $- carbon atom. Hence, if an $\alpha $- hydrogen is not present, this tautomerization will not occur.
In this problem, we can see that we have an $\alpha $- hydrogen, but it is not attached to an $s{{p}^{3}}$ hybridized carbon, so tautomerization should not be possible. But there is an exception to this rule that states that if a $\gamma $- hydrogen is attached to an $s{{p}^{3}}$ hybridized carbon, then the tautomerization can occur, and this $\gamma $- hydrogen will take the role of the $\alpha $- hydrogen.
In this problem, we can see that we do in fact have a $\gamma $- hydrogen that is attached to an $s{{p}^{3}}$ hybridized carbon. So, this compound will show keto-enol tautomerization using the $\gamma $- hydrogen. The product after the tautomerization will be:

Hence, the correct answer to this question is ‘C. ${{H}_{\gamma }}$’
Note:
Note that there are actually 2 $\gamma $- hydrogen atoms present on the para-carbon atom and any one of them can be used during the tautomerization. It is just due to convenience’s sake that only one of the two is denoted. Also note that the position of the double bonds change.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




