
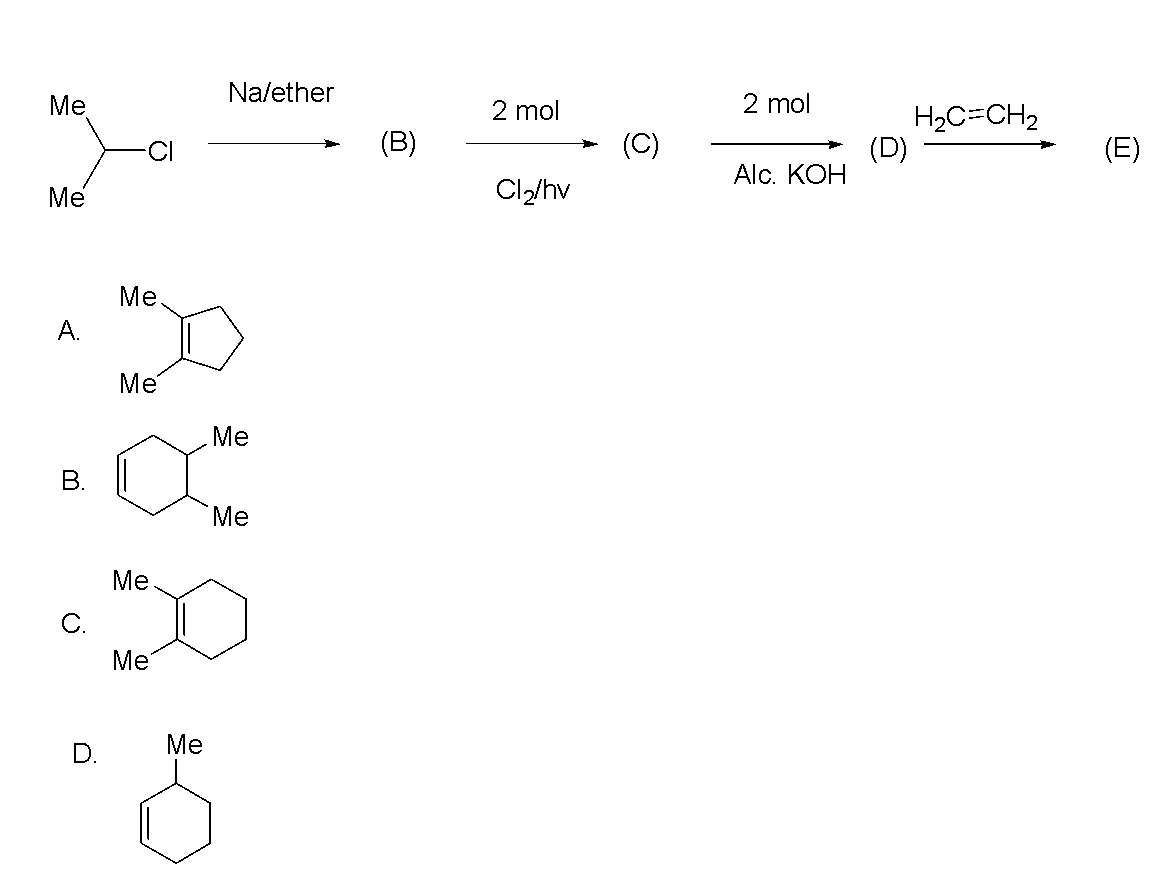
The final product (E) is

Answer
584.4k+ views
Hint: We must understand that in the first step of the reaction, a haloalkane treated with Na/ether is a typical Wurtz reaction, therefore the product B is dimer of the alkane part of the haloalkane. In the second step, the alkane obtained in the previous step is treated with 2 moles of Cl2/ light, here free radical substitution reaction occurs. The product obtained in this step is dihaloalkane (C). In the third step, C is treated with 2 moles of alcoholic KOH, this is a typical dehydrogenation reaction, i.e. removal of two moles of HCl occurs. As a result of this, we obtain a diene which undergoes Diels Alder cycloaddition reaction.
Complete step by step solution:
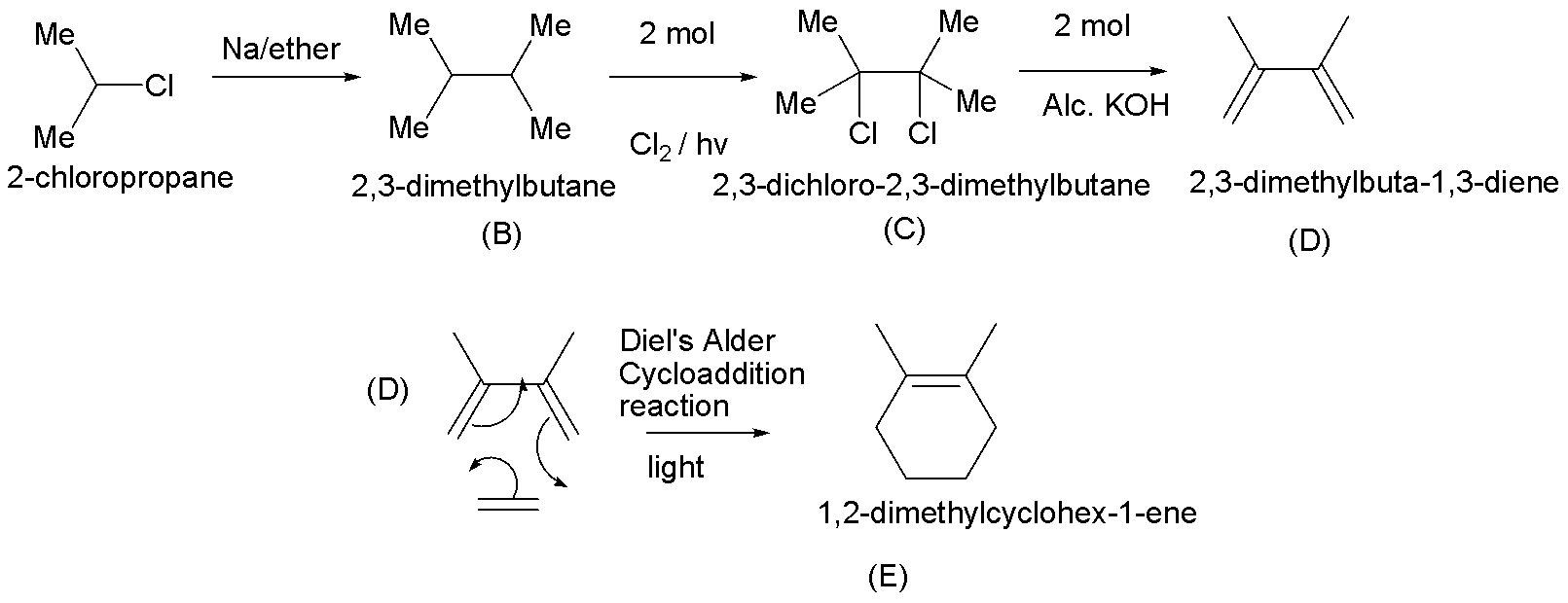
Step 1:- In this step, a haloalkane that is treated with Na/ether is a typical Wurtz coupling reaction. It is one of the oldest organic reactions, and produces the simple dimer derived from two equivalents of alkyl halide. Therefore the product obtained is 2,3-dimethylbutane (B).
Step 2:- Here, 2,3-dimethylbutane (B) is treated with 2 moles of chlorine in the presence of light. This is a free radical substitution reaction. The product obtained is 2,3-dichloro-2,3-dimethylbutane (C).
Step 3:- In this step, 2,3-dichloro-2,3-dimethylbutane undergoes dehydrohalogenation in the presence of strong bases such as alcohol \[{\text{KOH}}\]. Hence, the product in this reaction is 2,3-dimethylbuta-1,3-diene (D).
Step 4:- The obtained diene (D) undergoes Diels Alder cycloaddition reaction in the presence of light, hence the product (E) is 1,2-dimethylcyclohex-1-ene.
Hence, the correct option is C.
Note: 1. In Wurtz reaction, dry ether is used because sodium metal is used which is highly reactive with water. Therefore, we use dry solvent which does not react with sodium metal. Thus, dry ether is used.
2. In step 3, here dehydrohalogenation takes place as a type of \[{{\text{E}}_{\text{2}}}\]elimination reaction favoured in the presence of a strong base.
3. Step 4, cycloaddition reaction is the concerted bond formation between two independent pi-electron systems to form a new ring of atoms. Here, the Diels-Alder cycloaddition is classified as a [4+2] process because the diene has four pi-electrons that shift position in the reaction and the dienophile has two.
Complete step by step solution:

Step 1:- In this step, a haloalkane that is treated with Na/ether is a typical Wurtz coupling reaction. It is one of the oldest organic reactions, and produces the simple dimer derived from two equivalents of alkyl halide. Therefore the product obtained is 2,3-dimethylbutane (B).
Step 2:- Here, 2,3-dimethylbutane (B) is treated with 2 moles of chlorine in the presence of light. This is a free radical substitution reaction. The product obtained is 2,3-dichloro-2,3-dimethylbutane (C).
Step 3:- In this step, 2,3-dichloro-2,3-dimethylbutane undergoes dehydrohalogenation in the presence of strong bases such as alcohol \[{\text{KOH}}\]. Hence, the product in this reaction is 2,3-dimethylbuta-1,3-diene (D).
Step 4:- The obtained diene (D) undergoes Diels Alder cycloaddition reaction in the presence of light, hence the product (E) is 1,2-dimethylcyclohex-1-ene.
Hence, the correct option is C.
Note: 1. In Wurtz reaction, dry ether is used because sodium metal is used which is highly reactive with water. Therefore, we use dry solvent which does not react with sodium metal. Thus, dry ether is used.
2. In step 3, here dehydrohalogenation takes place as a type of \[{{\text{E}}_{\text{2}}}\]elimination reaction favoured in the presence of a strong base.
3. Step 4, cycloaddition reaction is the concerted bond formation between two independent pi-electron systems to form a new ring of atoms. Here, the Diels-Alder cycloaddition is classified as a [4+2] process because the diene has four pi-electrons that shift position in the reaction and the dienophile has two.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




