
The figures given show the location of atoms in three crystallographic planes in an f.c.c. lattice. Draw the unit cell for the corresponding structure and identify these planes in your diagram.
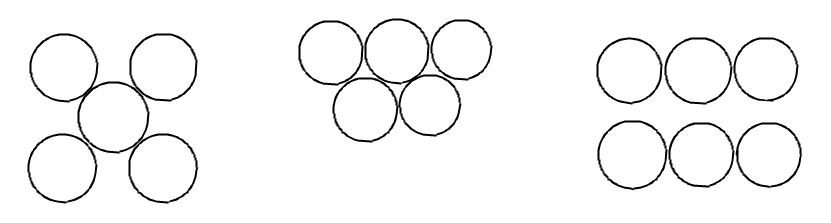
Answer
513k+ views
Hint: Crystallographic planes in the simple definition are a set of parallel and equally spaced planes that pass through the centers of atoms in a simple cube. In the fcc lattice there are atoms present at every corner of the cube along with one atom at the center of each face.
Complete answer: In the question above, we are given the location of atoms in a plane and we have to draw the unit cells that might give us these arrangements and identify those planes. We might have to imagine where these atoms may be present in a simple cube to draw the planes correctly. Let us try to draw by looking at the image one by one:
The first diagram looks like it is the face of one FCC lattice because it has four atoms at the corners and one at the center. Like in this figure:
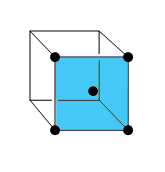
This plane is a face plane.
For the second diagram, you can say that it is not in a single face. The plane should pass the center of each face to get the diagram correctly. Let us try to draw a plane for this set of atoms. And the plane comes out to be like this:
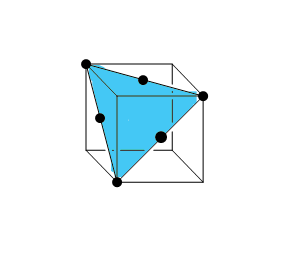
This is a triangular plane
Now for the third diagram it seems that the plane is a diagonal plane. The planes will pass through the center of the top and the bottom face. And the unit cell for this diagram would be:
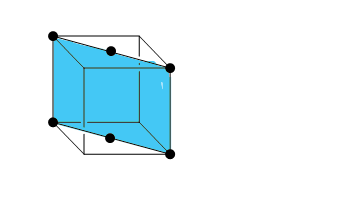
This plane is a diagonal face plane.
Note:
There is more than one plane of one type in a unit cell. Above only one plane is shown for the three types but there are more. The only thing you should be sure of is that the planes should pass through the center of each face because this is a fcc lattice.
Complete answer: In the question above, we are given the location of atoms in a plane and we have to draw the unit cells that might give us these arrangements and identify those planes. We might have to imagine where these atoms may be present in a simple cube to draw the planes correctly. Let us try to draw by looking at the image one by one:
The first diagram looks like it is the face of one FCC lattice because it has four atoms at the corners and one at the center. Like in this figure:

This plane is a face plane.
For the second diagram, you can say that it is not in a single face. The plane should pass the center of each face to get the diagram correctly. Let us try to draw a plane for this set of atoms. And the plane comes out to be like this:

This is a triangular plane
Now for the third diagram it seems that the plane is a diagonal plane. The planes will pass through the center of the top and the bottom face. And the unit cell for this diagram would be:

This plane is a diagonal face plane.
Note:
There is more than one plane of one type in a unit cell. Above only one plane is shown for the three types but there are more. The only thing you should be sure of is that the planes should pass through the center of each face because this is a fcc lattice.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




