
The energy profile of a reaction is used to show the successful collision of every molecule.
Which of the following is the correct order for an energy profile when reading the energy profile from left to right for a simple reaction?
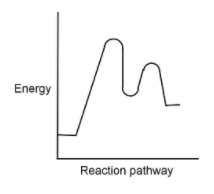
This question has multiple correct options.
A.Reactants, intermediate, activated complex, products.
B.Reactants, intermediate, activated complex, intermediate, products.
C.Reactants, activated complex, intermediate, products.
D.Reactants, activated complex products.

Answer
575.1k+ views
Hint: At the initial phase of reaction only reactant is present. Activity complexes and intermediate are highly energetic and very unstable, so they exist only for a shorter duration.
Complete step by step answer:
Collision theory was proposed by Max Trautz and William Lewis. As per this theory simple bimolecular gaseous reaction takes place when reactant molecules collide with each other. Total number of collisions which take place per unit volume per unit time is called collision frequency. Not every collision converts reactants into products, the collisions which do so are called effective collisions whose number is very less as compared to the total number of collisions. For a condition to be effective the following condition must be satisfied: the presence of threshold energy, activation energy and proper orientation.
According to diagram given in the question the correct order must be:
Reactant, activated complex, intermediate, activated complex and products.
This is because at the initial phase of the reaction only reactant is present. Now according to collision theory an activated complex is formed in between the reaction to form a product. Activated complex gets converted to the intermediate, if the reaction is having any intermediate. The intermediate then forms a product and an activated complex is formed in between.
Both option A and C can be considered to be correct.
Note:
Activated complex has the highest energy than any other entity during the reaction. There are some limitations of collision theory that it is applicable on gaseous reaction and molecular reaction in solution phase. This theory is applicable only in simpler reactions and fails to explain the complex mechanism. This theory considered reactant molecules as hard spheres and neglected their actual structures.
Complete step by step answer:
Collision theory was proposed by Max Trautz and William Lewis. As per this theory simple bimolecular gaseous reaction takes place when reactant molecules collide with each other. Total number of collisions which take place per unit volume per unit time is called collision frequency. Not every collision converts reactants into products, the collisions which do so are called effective collisions whose number is very less as compared to the total number of collisions. For a condition to be effective the following condition must be satisfied: the presence of threshold energy, activation energy and proper orientation.
According to diagram given in the question the correct order must be:
Reactant, activated complex, intermediate, activated complex and products.
This is because at the initial phase of the reaction only reactant is present. Now according to collision theory an activated complex is formed in between the reaction to form a product. Activated complex gets converted to the intermediate, if the reaction is having any intermediate. The intermediate then forms a product and an activated complex is formed in between.
Both option A and C can be considered to be correct.
Note:
Activated complex has the highest energy than any other entity during the reaction. There are some limitations of collision theory that it is applicable on gaseous reaction and molecular reaction in solution phase. This theory is applicable only in simpler reactions and fails to explain the complex mechanism. This theory considered reactant molecules as hard spheres and neglected their actual structures.
Recently Updated Pages
Complete reduction of benzene diazonium chloride with class 12 chemistry CBSE

How can you identify optical isomers class 12 chemistry CBSE

The coating formed on the metals such as iron silver class 12 chemistry CBSE

Metals are refined by using different methods Which class 12 chemistry CBSE

What do you understand by denaturation of proteins class 12 chemistry CBSE

Assertion Nitrobenzene is used as a solvent in FriedelCrafts class 12 chemistry CBSE

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

RNA and DNA are chiral molecules their chirality is class 12 chemistry CBSE




