
The end product D what is the reaction?
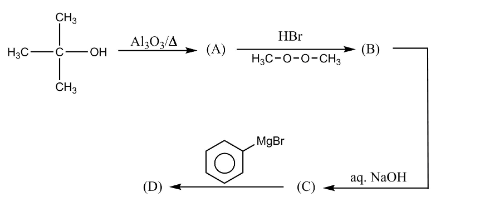

Answer
543.9k+ views
Hint: This question gives the knowledge about the dehydration reaction, addition reaction and Grignard’s reagent reaction. Dehydration reaction is the reaction in which release of water molecules takes place with the help of a dehydrating agent.
Complete step-by-step answer: It is a multistep reaction. A multistep reaction is the reaction which completes through various steps. In this reaction, at each step various name reactions are taking place.
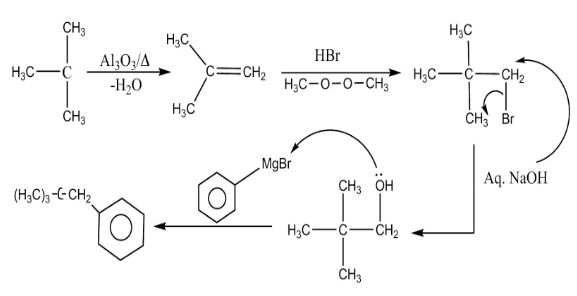
The detailed mechanism of the reaction is as follows:
In the first step a dehydration reaction is taking place. Dehydration reaction is the reaction in which release of water molecules takes place with the help of a dehydrating agent. In this step, tertiary butanol reacts with aluminum oxide in the presence of heat to form alkene.
In the second step, the resultant alkene undergoes addition reaction with hydrogen bromide in the presence of peroxide using anti-markovnikov rule. In this step the bromide group attaches to the less hindered site.
In the third step, elimination reaction takes place. In this step the bromide group gets eliminated by the action of aqueous sodium hydroxide.
In the fourth step, Grignard’s reaction takes place. In this step the negative charge present on the benzene ring forms a bond with the positive charge on the carbon atom of another molecule.
The end product $D$ of the reaction is
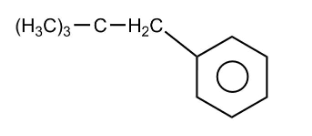
Hence, option $2$ is the correct option.
Note: Dehydrating reaction results in the removal of water molecules when two molecules react with each other. Elimination reactions generally leads to the formation of alkene by the removal of good leaving groups such as bromide, chloride and so forth.
Complete step-by-step answer: It is a multistep reaction. A multistep reaction is the reaction which completes through various steps. In this reaction, at each step various name reactions are taking place.
The detailed mechanism of the reaction is as follows:
In the first step a dehydration reaction is taking place. Dehydration reaction is the reaction in which release of water molecules takes place with the help of a dehydrating agent. In this step, tertiary butanol reacts with aluminum oxide in the presence of heat to form alkene.
In the second step, the resultant alkene undergoes addition reaction with hydrogen bromide in the presence of peroxide using anti-markovnikov rule. In this step the bromide group attaches to the less hindered site.
In the third step, elimination reaction takes place. In this step the bromide group gets eliminated by the action of aqueous sodium hydroxide.
In the fourth step, Grignard’s reaction takes place. In this step the negative charge present on the benzene ring forms a bond with the positive charge on the carbon atom of another molecule.

The end product $D$ of the reaction is

Hence, option $2$ is the correct option.
Note: Dehydrating reaction results in the removal of water molecules when two molecules react with each other. Elimination reactions generally leads to the formation of alkene by the removal of good leaving groups such as bromide, chloride and so forth.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




