
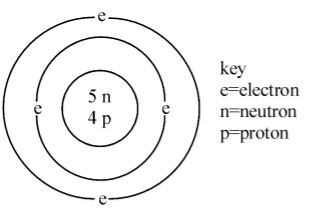
The diagram given below represents the atom of an element. Which symbol gives the above information?
A.${{_{4}}^{9}}Be$
B.${{_{4}}^{9}}F$
C.${{_{4}}^{9}}B$
D.${{_{4}}^{9}}O$

Answer
582.9k+ views
Hint: Atomic number is defined as the total no of protons or electrons present in the nucleus or in the orbits of the atoms.
Mass number is defined as the total no of neutrons and protons present in the nucleus of the atom.
Complete answer:
The term atomic number is conventionally denoted by the symbol Z it indicates the number of protons present in the nucleus of an atom, which is also equal to the number of electrons in an uncharged atom. The number of neutrons in the atom is represented by the neutron number (N). It is written below the element
Similarly the term mass no is conventionally denoted by the symbol M it indicates the no of protons and neutrons present in the nucleus of the atom. The number of neutrons is denoted by the mass no(M). It is written above the element.
In the above diagram there are 4 protons so the atomic no is 4 and written below.
And there are 5 neutrons and 4 protons ,there are a total 9 protons and neutrons so the mass no is 9 and written above.
As the element with atomic no 4 is beryllium.
Therefore the correct option is A.
Note:
Beryllium is a chemical element with the symbol Be and has an atomic number 4. It is an apparently rare element in the universe, usually occurring as a product of the disintegration of larger atomic nuclei that have collided with cosmic rays. Within the cores of stars, beryllium is depleted as it is fused into other heavier elements.
Mass number is defined as the total no of neutrons and protons present in the nucleus of the atom.
Complete answer:
The term atomic number is conventionally denoted by the symbol Z it indicates the number of protons present in the nucleus of an atom, which is also equal to the number of electrons in an uncharged atom. The number of neutrons in the atom is represented by the neutron number (N). It is written below the element
Similarly the term mass no is conventionally denoted by the symbol M it indicates the no of protons and neutrons present in the nucleus of the atom. The number of neutrons is denoted by the mass no(M). It is written above the element.
In the above diagram there are 4 protons so the atomic no is 4 and written below.
And there are 5 neutrons and 4 protons ,there are a total 9 protons and neutrons so the mass no is 9 and written above.
As the element with atomic no 4 is beryllium.
Therefore the correct option is A.
Note:
Beryllium is a chemical element with the symbol Be and has an atomic number 4. It is an apparently rare element in the universe, usually occurring as a product of the disintegration of larger atomic nuclei that have collided with cosmic rays. Within the cores of stars, beryllium is depleted as it is fused into other heavier elements.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction

State the laws of reflection of light




