
The d-electron configurations of \[{\text{C}}{{\text{r}}^{{\text{2 + }}}}{\text{,M}}{{\text{n}}^{{\text{2 + }}}}{\text{,F}}{{\text{e}}^{{\text{2 + }}}}{\text{ and C}}{{\text{o}}^{{\text{2 + }}}}\] are \[{{\text{d}}^{\text{4}}}{\text{,}}{{\text{d}}^{\text{5}}}{\text{,}}{{\text{d}}^{\text{6}}}{\text{ and }}{{\text{d}}^{\text{7}}}\]respectively. Which one of the following will exhibit minimum paramagnetic behaviour?
(Atomic numbers of Cr = 24, Mn = 25, Fe = 26, Co =27)
A.\[{{\text{[Mn(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{{\text{2 + }}}}\]
B.\[{{\text{[Fe(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{{\text{2 + }}}}\]
C.\[{{\text{[Co(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{{\text{2 + }}}}\]
D.\[{{\text{[Cr(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{{\text{2 + }}}}\]
Answer
589.5k+ views
Hint: Water is a strong ligand thus no pairing of electrons takes place while filling of electrons in orbitals. The presence of unpaired electrons leads to paramagnetic behaviour.
Complete step by step answer:
In all the given complexes, the oxidation state of metals such as \[{\text{Cr,Mn,Fe and Co}}\] is +2 thus the outer shell electronic configuration becomes \[{{\text{d}}^{\text{4}}}{\text{,}}{{\text{d}}^{\text{5}}}{\text{,}}{{\text{d}}^{\text{6}}}{\text{ and }}{{\text{d}}^{\text{7}}}\] respectively. The 3d orbital diagram for each complex can be depicted as below:
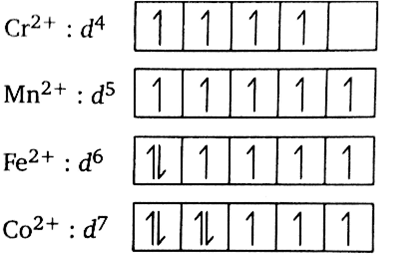
The unshared pair of electrons simply refers to the pair of valence electrons which are not shared during a covalent bonding. This term is sometimes called a lone pair of electrons or non-bonding pair of electrons. The presence of unpaired electrons indicates the paramagnetic behaviour. Greater the number of electrons greater the paramagnetic behaviour. Magnetic moment can be calculated from the spin only formula,
${{\mu = }}\sqrt {{\text{n(n + 2)}}} {\text{ BM}}$ (BM- Bohr magneton, unit of magnetic moment)
where n is the number of unpaired electrons. More the unpaired electrons more the magnetic moment.
In the given complexes, \[{{\text{[Mn(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{{\text{2 + }}}}\]has the maximum number of unpaired electrons (5 unpaired electrons) thus that complex will exhibit maximum paramagnetic behaviour.
While \[{{\text{[Co(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{{\text{2 + }}}}\]has the minimum number of unpaired electrons (3 unpaired electrons) thus that complex will exhibit minimum paramagnetic behaviour.
So, the correct option is C.
Note:
In case of absence of unpaired electrons, there will be no paramagnetic behaviour instead the complex will exhibit diamagnetic behaviour.
Complete step by step answer:
In all the given complexes, the oxidation state of metals such as \[{\text{Cr,Mn,Fe and Co}}\] is +2 thus the outer shell electronic configuration becomes \[{{\text{d}}^{\text{4}}}{\text{,}}{{\text{d}}^{\text{5}}}{\text{,}}{{\text{d}}^{\text{6}}}{\text{ and }}{{\text{d}}^{\text{7}}}\] respectively. The 3d orbital diagram for each complex can be depicted as below:

The unshared pair of electrons simply refers to the pair of valence electrons which are not shared during a covalent bonding. This term is sometimes called a lone pair of electrons or non-bonding pair of electrons. The presence of unpaired electrons indicates the paramagnetic behaviour. Greater the number of electrons greater the paramagnetic behaviour. Magnetic moment can be calculated from the spin only formula,
${{\mu = }}\sqrt {{\text{n(n + 2)}}} {\text{ BM}}$ (BM- Bohr magneton, unit of magnetic moment)
where n is the number of unpaired electrons. More the unpaired electrons more the magnetic moment.
In the given complexes, \[{{\text{[Mn(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{{\text{2 + }}}}\]has the maximum number of unpaired electrons (5 unpaired electrons) thus that complex will exhibit maximum paramagnetic behaviour.
While \[{{\text{[Co(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{{\text{2 + }}}}\]has the minimum number of unpaired electrons (3 unpaired electrons) thus that complex will exhibit minimum paramagnetic behaviour.
So, the correct option is C.
Note:
In case of absence of unpaired electrons, there will be no paramagnetic behaviour instead the complex will exhibit diamagnetic behaviour.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




